SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold Immunochromatography)
Product nameSARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold Immunochromatography)Model 1 test/kit; 5 tests/kit; 10 tests/kit; 25 tests/kit; 50 tests/kit.Intended Use The product is intended for the qualitative detection of antigen against SARS-CoV-2 in clinical samples (nasal swab).
Summary
Coronavirus, as a large virus family, is a single positive-stranded RNA virus with an envelope. The virus is known to cause major illnesses such as colds, Middle East Respiratory Syndrome (MERS), and Severe Acute Respiratory Syndrome (SARS). The core protein of SARS-CoV-2 is the N protein (Nucleocapsid), which is a protein component located inside the virus. It is relatively conserved among β-coronaviruses and is often used as a tool for the diagnosis of coronaviruses. ACE2, as a key receptor for SARS-CoV-2 to enter cells, is of great significance for the research of viral infection mechanisms.Principle The current test card is based on the specific antibody-antigen reaction and immune analysis technology.The test card contains colloidal gold-labeled SARS-CoV-2 N protein monoclonal antibody which ispre-coated on the combination pad, matched SARS-CoV-2 N protein monoclonal antibody immobilized on the Test area (T) and corresponding antibody in the quality control area (C).During testing, the N protein in the sample combines with the colloidal gold-labeled SARS-CoV-2 Nprotein monoclonal antibody which is pre-coated on the combination pad. The conjugates migrate upward under the capillary effect and are subsequently captured by the N protein monoclonal antibody immobilized in the test area (T). The higher the contents of N protein in the sample, the more the conjugates capture and the darker the color in the test area is. If there is no virus in the sample or the virus content is lower than the detection limit, then there is no color demonstrated in the test area (T). Regardless of the presence or absence of the virus in the sample, a purple stripe will appear in the quality control area (C). The purple stripe in the quality control area (C) is a criterion for the judgment of whether or not there is enough sample and whether or not the chromatography procedure is normal.
Component
The product consists of test cards, Instructions for use, sample treatment solutions. And in each test card bag, includes one SARS-CoV-2 antigen detection card and one package of desiccants.
|
Model |
Test card | Instructions for use |
Sample treatment solution |
| I test/kit | I test | I | 1mlx1 |
| 5 tests/kit | 5 tests | I | 1mIx’ |
| 10tests/kit | 10 tests | I | 2m1x1 |
| 25 tests/kit | 25 tests | I | 3m1x2 |
| 50 tests/kit | 50 tests | I | 5m1x2 |
|
Each test card bag contains one test card and one package of desiccants. |
The test card consists of a gold standard mat (coated with colloidal gold-labeled SARS-CoV-2 N protein monoclonal antibody), sample mat, nitrocellulose membrane (Test area (T) is coated with a SARS-CoV-2 N protein monoclonal antibody; the quality control area (C) is coated with goat anti-mouse antibody), absorbing paper, and hydrophobic stiff card.Storage and Stability It should be stored at 4°C~ 30°C, be kept dry and away from sunlight. The shelf life is 12 months.For per test card, it should be used within 1 hour after unsealing.Production Date and Expiration date are shown in the package label.Sample Requirements The product is used to test the human nasal swab sample.Sample collection: During the collection procedures for samples, take care to make proper protection, and avoid direct contact with the sample. In case of accidental contact, disinfection treatment should be carried out in time and necessary measures should be taken.Nasal swab sample: gently and slowly insert the swab into the nasopharynx through the nasal cavity. When resistance is encountered, the swab will arrive at the posterior nasopharynx. After a few seconds of suction, gently rotate the swab, then take out the swab to obtain the nasal swab sample.Sample preservation: after sample collection, please complete the test within 1 hour.The sample should come to room temperature before testing.
Test Method
Please read the instruction for use carefully before performing the test. Before testing, restore the reagents and sample to room temperature.
- During sampling, the swab head should be completely inserted into the nasal cavity and gently rotated 5 times. After removal, the swab head should be sampled in the other nasal cavity, in the same way, to ensure that enough samples are taken.
- Before the test, the double-sided adhesive protective layer should be removed in advance to prevent liquid splashing. If the double-sided adhesive protective layer is torn off after adding diluent, it is easy to cause liquid splashing.
- Add 6 drops of the diluent into well A. Do not drop the diluent into the other wells.
- During the test, the test card should be placed on the horizontal desktop. The test card should be fixed and do not remove test card.
- After covering the left side, gently press the adhesive position to make the two sides completely fit and start timing. Wait until the purple band appears. The test result should be read within 15-20 minutes.
The Explanation of the Testing Results
• Positive (+): There appear purple stripes in both the quality control area (C) and either test area (T).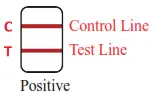 • Negative (-): There is only one purple stripe in the quality control area (C), and without purple stripe in either test area (T).
• Negative (-): There is only one purple stripe in the quality control area (C), and without purple stripe in either test area (T). • Invalid: There is no purple stripe in the quality control area (C), or there is a blue stripe in the quality controlarea (C), indicating incorrect operating procedures or the test card has already deteriorated. Under this condition, it must read the instruction for use again carefully and then use the new test card to test again. If the problem still exists, stop using the products with the same lot number and contact the local suppliers immediately.
• Invalid: There is no purple stripe in the quality control area (C), or there is a blue stripe in the quality controlarea (C), indicating incorrect operating procedures or the test card has already deteriorated. Under this condition, it must read the instruction for use again carefully and then use the new test card to test again. If the problem still exists, stop using the products with the same lot number and contact the local suppliers immediately. Limitation of Procedure
Limitation of Procedure
- The test results of this product should be comprehensively judged by the physician in combination with other clinical information, and should not be used as the only criterion;
- The product is used to test the SARS-CoV-2 antigen of the clinical sample.
Product Performance Index
- Physical Property1.1 AppearanceThe test card should be clean and integral, no burrs, no damage, no pollution; the material should be firmlyattached; the label should be clear and not damaged. The sample dilution should be clear without impuritiesand flocs.1.2 Liquid migration speedThe liquid migration speed should be no less than 10mm/min.1.3 Membrane Strip WidthThe membrane strip width of the testing card should be≥2.5mm.1.4 The preparation quantity of the diluent for the samplesThe volume of the diluents for the sample is no less than the indicated value.
- Detection LimitFor the detection of sensitivity reference material, the positive detection rate should be no less than 90%.
- Negative reference products compliance rateFor the detection of negative reference material, the negative detection rate should be 100%.
- Positive reference products compliance rateFor the detection of positive reference material, the positive detection rate should be 100%.
- RepeatabilityFor the detection of enterprise reference material P2 and P4, the results should be positive and the colorrendering should be uniform.
- Cross-reactivityCross-reactivity: This test device has no cross-reactivity with endemicity human coronavirus OC43, influenza a virus, influenza B virus, respiratory syncytial virus, adenovirus, EB virus, measles virus, cytomegalovirus, rotavirus, Norovirus, mumps virus, varicella-zoster virus, mycoplasma pneumonia, Human metapneumovirus.
- Clinical Performance210 clinical samples based on the nucleic acid detection method (PCR) test results were obtained for testing, including 75 positive and 135 negative samples. The SARS-CoV-2 Antigen Rapid Test Kit was compared with the nucleic acid method (PCR) using the collected clinical samples. The results were summarized in the table below:
|
SARS-CoV-2 Antigen RapidTest Kit |
Nucleic acid detection method (PCR) |
|
| Positive | Negative | |
| Positive | 69 | 1 |
| Negative | 6 | 134 |
| Diagnostic Specificity | 92.00% (95%CI: 83.63%-96.28%) | / |
| Diagnostic Specificity | / | 99.26% (95%CI: 95.92%-99.87%) |
Precautions
- The test is only suitable for professionals to use in vitro auxiliary diagnosis. Do not use expired products.
- Do not freeze or use after the expiration date (see the packaging for the expiration date).
- Avoid excessive temperature and humidity in the experimental environment. The reaction temperature should be 15-30 ° C and the humidity should be below 70%.
- The test card bag contains desiccant, and it should not be taken orally.
- When testing, please wear protective clothing, a medical mask, gloves, and goggles.
- Do not use the test card with broken single packaging, unclear marks, and past the expiration date.
- Dispose of used specimens, test cards, and other waste in accordance with relevant local laws and regulations.
- The test card should be used within 1 hour after being taken out of the aluminum foil bag.
- The users should take samples according to the requirements of IFU.
- Before the test, the double-sided adhesive protective layer should be removed in advance to prevent liquidsplashing. If the double-sided adhesive protective layer is torn off after adding diluent, it is easy to cause liquid splashing.
- Do not drop the diluent into the wrong well.
- During the test, the test card should be placed on the horizontal desktop. The test card should be fixed and do not remove test card.
Explanation of Symbols
 |
DO NOT USE IF PACKAGE IS DAMAGED | CONSULTINSTRUCTIONSFOR USE | |
| DO NOT REUSE | USE-BY DATE | ||
| TEMPERATURE LIMIT | DATE OF MANUFACTURER | ||
| MANUFACTURER | BATCH CODE | ||
| KEEP AWAY FROM
SUNLIGHT |
KEEP DRY | ||
| IN VITRODIAGNOSTICMEDICAL DEVICE | CE MARK | ||
| AUTHORIZEDREPRESENTATIVEIN THE EUROPEANCOMMUNITY |
Basic Information
![]()
![]()
![]()
![]()
![]()
![]()
Lepu Medical (Europe) Cooperatief U.A.Abe Lenstra Boulevard 36, 8448 JB, Heerenveen, The NetherlandsTel: +31-515-573399 Fax: +31-515-760020
Approval Date and Revision Date of the InstructionApproved on 2nd, Sept., 2020;Version number: CE-InCG27 REV.05
References
[xyz-ips snippet=”download-snippet”]

