ABBOTT BinaxNOW Covid-19 Antigen Test Instructions
ABBOTT BinaxNOW Covid-19 Antigen Test Instructions
PROCEDURE CARD
For Use Under an Emergency Use Authorization (EUA) Only.The BinaxNOW COVID-19 Ag Card is a lateral flow immunoassay for the qualitative detection of the nucleocapsid protein antigen to SARS-CoV-2 directly from anterior nasal (nares) swab specimens collected from individuals who are suspected of COVID-19 by their healthcare provider within seven days of the onset of symptoms.IMPORTANT: See Product Insert, including QC section, for complete use instructions, warnings, precautions and limitations.False negative results may occur if specimens are tested past 1 hour of collection. Specimens should be tested as quickly as possible after specimen collection. Open the test card just prior to use, lay it flat, and perform assay as follows.
Part 1 – Sample Test Procedure
Patient Samples require 6 drops of Extraction Reagent
- Correct
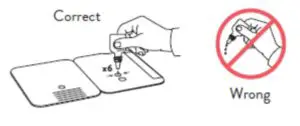
Hold Extraction Reagent bottle vertically. Hovering 1/2inch above the TOP HOLE, slowly add 6 DROPS to the TOP HOLE of the swab well. DO NOT touch the cardwith the dropper tip while dispensing.
2.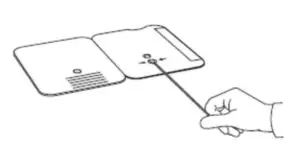
Insert sample or control swab into BOTTOM HOLE andfirmly push upwards so that the swab tip is visible in theTOP HOLE.
3.

Rotate (twirl) swab shaft 3 times CLOCKWISE (tothe right). Do not remove swab.
4.
Used test cards should be discarded as Biohazard waste according to Federal, State and local regulatory requirements.
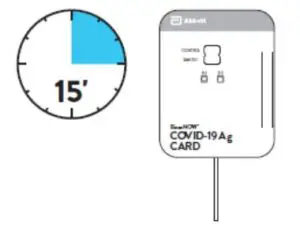
Peel off adhesive liner from the right edge of the test card.Close and securely seal the card. Read result in the window15 minutes after closing the hard. In order to ensure propertest performance, it is important to read the result promptly at 15 minutes, and not before. Results should not be read after 30 minutes.
In the USA, this product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories; use by laboratories certifed under the CLIA, 42 U.S.C. §263a, that meet requirements to perform moderate, high or waivedcomplexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certifcate of Waiver, Certifcate of Compliance, or Certifcate of Accreditation. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. In the USA, – this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of the virus that causes COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revokedsooner.
Part 2 – Result Interpretation
Negative Result
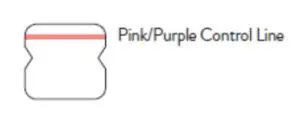

A negative specimen will give a single pink/purple colored ControlLine in the top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected. Negative results should be treated as presumptive and confirmation with a molecularassay, if necessary, for patient management, may be performed.
Positive Result


A positive specimen will give two pink/purple colored lines.This means that COVID-19 antigen was detected. Specimenswith low levels of antigen may give a faint Sample Line. Any visible pink/purple colored line is positive.
Invalid Result
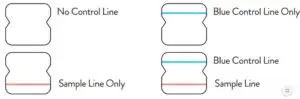

If no lines are seen, or if just the Sample Line is seen, the assay isinvalid. Invalid tests should be repeated.
Procedure for External Quality Control Testing
External Controls require 8 drops of Extraction ReagentHold
- Extraction Reagent bottle vertically. Hovering 1/2 inchabove the TOP HOLE, slowly add 8 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.
- Follow Steps 2 – 4 of the Test Procedure shown.
BinaxNOWTM COVID-19 Ag CARD
For Use Under an Emergency Use Authorization (EUA) OnlyFor use with anterior nasal (nares) swab specimens For in vitro Diagnostic Use Only Rx Only
INTENDED USEThe BinaxNOWTM COVID-19 Ag Card is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal (nares) swabs from individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point ofCare (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.The BinaxNOWTM COVID-19 Ag Card does not differentiate between SARS-CoV and SARS-CoV-2.Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in anterior nasal (nares) swabs during the acute phase of infection. Positiveresults indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do notrule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all results to the appropriate public health authorities.Negative results should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed. Negative results do not rule out SARSCoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in thecontext of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.The BinaxNOWTM COVID-19 Ag Card is intended for use by medical professionals or trained operators who are proficient in performing rapid lateral flow tests. BinaxNOWTM COVID-19 AgCard is only for use under the Food and Drug Administration’s Emergency Use Authorization.
SUMMARY AND EXPLANATION OF THE TEST
Coronaviruses are a large family of viruses which may cause illness in animals or humans. SARSCoV-2 is an enveloped, single-stranded RNA virus of the β genus. The virus can cause mild to
severe respiratory illness and has spread globally, including the United States.BinaxNOWTM COVID-19 Ag Card is a rapid lateral flow immunoassay for the qualitative detection and diagnosis of SARS-CoV-2 directly from nasal swabs, without viral transport media. The BinaxNOWTM COVID-19 Ag Card kit contains all components required to carry out an assay for SARS-CoV-2.
PRINCIPLES OF THE PROCEDURE
The BinaxNOWTM COVID-19 Ag Card is an immunochromatographic membrane assay that useshighly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab specimens. SARS-CoV-2 specific antibodies and a control antibody are immobilized onto amembrane support as two distinct lines and combined with other reagents/pads to construct a test strip. This test strip and a well to hold the swab specimen are mounted on opposite sides ofa cardboard, book-shaped hinged test card. To perform the test, a nasal swab specimen is collected from the patient, 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab well. The patient sample is inserted into the test card through the bottom hole of the swab well, and firmly pushed upwards until the swab tip is visible through the top hole. The swab is rotated 3 times clockwise and the card is closed, bringing the extracted sample into contact with the test strip. Test results areinterpreted visually at 15 minutes based on the presence or absence of visually detectable pink/purple colored lines. Results should not be read after 30 minutes.
REAGENTS AND MATERIALS
Materials ProvidedTest Cards (40): A cardboard, book-shaped hinged test card containing the test stripExtraction Reagent (1): Bottle containing 7.5 mL of extraction reagentNasal Swabs (40): Sterile swabs for use with BinaxNOWTM COVID-19 Ag Card testPositive Control Swab (1) : Non-infectious recombinant SARS-CoV-2 nucleocapsid antigen dried onto a swabNegative Control Swab: The use of a sterile patient swab ensures appropriate negative results are obtainedProduct Insert (1)Procedure Card (1)Materials Required but not ProvidedClock, timer or stopwatchMaterials Available as an Optional AccessorySwab Transport Tube Accessory Pack
PRECAUTIONS
- For in vitro diagnostic use.
- This product has not been FDA cleared or approved; but has been authorized by FDAunder an EUA for use by laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform moderate, high or waived complexity tests and at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
- Federal Law restricts this device to sale by or on the order of a licensed practitioner (US only).
- This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens
- This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
- Laboratories within the United States and its territories are required to report all results to the appropriate public health laboratories.
- 7. Treat all specimens as potentially infectious. Follow universal precautions when handling samples, this kit and its contents.
- Proper sample collection, storage and transport are essential for correct results.
- Leave test card sealed in its foil pouch until just before use. Do not use if pouch is damaged or open.
- Do not use kit past its expiration date.
- Do not mix components from different kit lots.
- Do not reuse the used test card.
- Inadequate or inappropriate sample collection, storage, and transport may yield false test results.
- Do not store or test specimens in viral transport media, as it may result in false positive or false negative results.
- All components of this kit should be discarded as Biohazard waste according to Federal, State and local regulatory requirements.
- Solutions used to make the positive control swab are non-infectious. However, patient samples, controls, and test cards should be handled as though they could transmit disease. Observe established precautions against microbial hazards during use and disposal.
- Wear appropriate personal protection equipment and gloves when running each test and handling patient specimens. Change gloves between handling of specimens suspected of COVID-19.
- INVALID RESULTS can occur when an insufficient volume of extraction reagent is added to the test card. To ensure delivery of adequate volume, hold vial vertically, ½ inch above the swab well, and add drops slowly.
- False Negative results can occur if the sample swab is not rotated (twirled) prior to closing the card.
- Swabs in the kit are approved for use with BinaxNOWTM COVID-19 Ag Card. Do not use other swabs.
- The Extraction Reagent packaged in this kit contains saline, detergents and preservatives that will inactivate cells and virus particles. Samples eluted in this solution are not suitable for culture.
- Do not store the swab after specimen collection in the original paper packaging, if storage is needed use a plastic tube with cap.
STORAGE AND STABILITY
Store kit at 2-30°C. The BinaxNOWTM COVID-19 Ag Card kit is stable until the expiration date marked on the outer packaging and containers. Ensure all test components are at room temperature before use.
QUALITY CONTROL
BinaxNOWTM COVID-19 Ag Card has built-in procedural controls. For daily quality control, Abbott suggests that you record these controls for each test run.
Procedural Controls:
- The pink-to-purple line at the “Control” position is an internal procedural control. If the test flows and the reagents work, this line will always appear.
- The clearing of background color from the result window is a negative background control. The background color in the window should be light pink to white within 15 minutes. Background color should not hinder reading of the test.
External Positive and Negative Controls:Good laboratory practice suggests the use of positive and negative controls to ensure that test reagents are working and that the test is correctly performed. BinaxNOWTM COVID-19 Ag Cardkits contain a Positive Control Swab and Sterile Swabs that can be used as a Negative Control Swab. These swabs will monitor the entire assay. Test these swabs once with each new shipmentreceived and once for each untrained operator. Further controls may be tested in order to conform with local, state and/or federal regulations, accrediting groups, or your lab’s standard QualityControl procedures.
If the correct control results are not obtained, do not perform patient tests or report patient results. Contact Technical Support during normal business hours before testing patient specimens.
SPECIMEN COLLECTION AND HANDLING
Test specimens immediately after collection for optimal test performance. Inadequate specimen collection or improper sample handling/storage/transport may yield erroneous results. Refer tothe CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019 nCoV/lab/guidelines-clinical-specimens.html
Anterior Nasal (Nares) SwabOnly the swab provided in the kit is to be used for nasal swab collection.To collect a nasal swab sample, carefully insert the entire absorbent tip of the swab (usually ½ to ¾ of an inch (1 to 1.5 cm) into the nostril. Firmly sample the nasal wall by rotating the swab in a circular path against the nasal wall 5 times or more for a total of15 seconds, then slowly remove from the nostril. Using the same swab, repeat sample collection in the other nostril.
SPECIMEN TRANSPORT AND STORAGE
Do not return the nasal swab to the original paper packaging.For best performance, direct nasal swabs should be tested as soon as possible after collection. If immediate testing is not possible, and to maintain best performance and avoid possible contamination, it is highly recommended the nasal swab is placed in a clean, unused plastic tube labeled with patient information, preserving sample integrity, and capped tightly at room temperature (15-30°C) for up to (1) hour prior to testing. Ensure the swab fits securely within the tube and the cap is tightly closed. If greater than 1 hour delay occurs, dispose of sample. A new sample must be collected for testing.
TEST PROCEDUREProcedure for Patient Specimens
Open the test card just prior to use, lay it flat, and perform assay as follows. The test card must be flat when performing testing, do not perform testing with the test card in any other position.
- Hold Extraction Reagent bottle vertically. Hovering 1/2 inch above the TOP HOLE, slowly add 6 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.



- Insert sample into BOTTOM HOLE and firmly push upwards so that the swab tip is visible in the TOP HOLE.


- Rotate (twirl) swab shaft 3 times CLOCKWISE (to the right). Do not remove swab.


Note: False negative results can occur if the sample swab is not rotated (twirled) prior to closing the card.
4. Peel off adhesive liner from the right edge of the test card. Close and securely seal the card. Read result in the window 15 minutes after closing the card. In order to ensure proper test performance, it is important to read the result promptly at 15 minutes, and not before. Results should not be read after 30 minutes.



Note: False negative results can occur if test results are read before 15 minutes.Note: When reading test results, tilt the card to reduce glare on the result window if necessary. Individuals with color-impaired vision may not be able to adequately interpret test results.
Procedure for BinaxNOW™ Swab Controls
Open the test card just prior to use, lay it flat, and perform assay as follows.
- Hold Extraction Reagent bottle vertically Hovering 1/2 inch above the TOP HOLE, slowly add 8 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.



- Follow Steps 2 – 4 of the Test Procedure for Patient Specimens.
RESULT INTERPRETATION
Note: In an untested BinaxNOW COVID-19 Ag Card there will be a blue line present at the Control Line position. In a valid, tested device, the blue line washes away and a pink/purple line appears, confirming that the sample has flowed through the test strip and the reagents are working. If the blue line is not present at the Control Line position prior to running the test, do not use and discard the test card.
Negative

A negative specimen will give a single pink/purplecolored Control Line in the top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected.
Positive


A positive specimen will give two pink/purple colored lines. This means that COVID-19 antigen was detected. Specimens with low levels of antigen may give a faint Sample Line. Any visible pink/purple colored line is positive.
Invalid


If no lines are seen, if just the Sample Line is seen, or the Blue Control Line remains blue, the assay is invalid. Invalid tests should be repeated.
LIMITATIONS
- This test detects both viable (live) and non-viable, SARS-CoV, and SARS-CoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- The performance of the BinaxNOW™ COVID-19 Ag Card was evaluated using the procedures provided in this product insert only. Modifications to these procedures may alter theperformance of the test.
- False negative results may occur if a specimen is improperly collected, transported, or handled.
- False results may occur if specimens are tested past 1 hour of collection. Specimens should be test as quickly as possible after specimen collection.
- False negative results may occur if inadequate extraction buffer is used (e.g., <6 drops).
- False negative results may occur if specimen swabs are not twirled within the test card.
- False negative results may occur if swabs are stored in their paper sheath after specimen collection.
- Positive test results do not rule out co-infections with other pathogens.
- False negative results are more likely after eight days or more of symptoms.
- Positive test results do not differentiate between SARS-CoV and SARS-CoV-2.
- Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
- The presence of mupirocin may interfere with the BinaxNOW™ COVID-19 Ag test and may cause false negative results.
- Negative results should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed.
- If the differentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local public health departments, is required.
The BinaxNOW™ COVID-19 Ag Card Letter of Authorization, along with the authorized Fact Sheet for Healthcare Providers, the authorized Fact Sheet for Patients, and authorized labeling are available on the FDA website: https://www.fda.gov/medical devices/coronavirus-disease2019-covid-19-emergency-use- uthorizations-medical-devices/vitro-diagnostics-euas. However, to assist clinical laboratories using the BinaxNOW™ COVID-19 Ag Card, the relevant
Conditions of Authorization are listed below:
- Authorized laboratories1 using your product must include with test result reports, all authorized Fact Sheets. Under exigent circumstances, other appropriate methods for disseminating these Fact Sheets may be used, which may include mass media.
- Authorized laboratories using your product must use your product as outlined in the “BinaxNOW™ COVID-19 Ag Card” Instructions for Use. Deviations from the authorized procedures, including the authorized instruments, authorized clinical specimen types, authorized control materials, authorized other ancillary reagents and authorized materials required to use your product are not permitted.
- Authorized laboratories that receive your product must notify the relevant public health authorities of their intent to run your product prior to initiating testing.
- Authorized laboratories using your product must have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
- Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: CDRH [email protected]) and Abbott Diagnostics Scarborough, Inc. (via email: [email protected], or via phone by contacting Abbott Diagnostics Scarborough, Inc. Technical Service at 1-800-257-9525) any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of your product of which they become aware.
- All operators using your product must be appropriately trained in performing and interpreting the results of your product, use appropriate personal protective equipment when handling this kit, and use your product in accordance with the authorized labeling.
- Abbott Diagnostics Scarborough, Inc., authorized distributors, and authorized laboratories using your product must ensure that any records associated with this EUA are maintained until otherwise notified by FDA. Such records will be made available to FDA for inspection upon request.
1 The letter of authorization refers to, “Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform high, moderate, or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.” as “authorized laboratories.”
PERFORMANCE CHARACTERISTICSCLINICAL PERFORMANCE
Clinical performance characteristics of BinaxNOW™ COVID-19 Ag Card was evaluated in a multisite prospective study in the U.S in which patients were sequentially enrolled and tested. A totalof ten (10) investigational sites throughout the U.S. participated in the study. Testing was performed by operators with no laboratory experience and who are representative of the intended users at CLIA waived testing sites. In this study testing was conducted by sixty-two (62) intended users. To be enrolled in the study, patients had to be presenting at the participating study centers with suspected COVID-19. Patients who presented within 7 days of symptom onset were included in the initial primary analysis. Two nasal swabs were collected from patients and tested using theBinaxNOW™ COVID-19 Ag Card at all study sites. An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-CoV-2 was utilized as the comparator method for this study. At all sites, one nasal swab was tested directly in the BinaxNOW™ COVID-19 Ag Card test according to product instructions and the other swab was eluted in viral transport media (VTM). Swabs were randomly assigned to testing with the BinaxNOW or RT-PCR testing and were testedby minimally trained operators who were blinded to the RT-PCR test result. All sites shipped the VTM sample to a central testing laboratory for RT-PCR.External control testing, using BinaxNOW™ COVID-19 Ag Card Positive and Negative Controls,was performed prior to sample testing each day, at all study sites.The performance of BinaxNOW™ COVID-19 Ag Card was established with 460 nasal swabs collected from individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW™ COVID-19 Ag Card Performance within 7 days of symptom onset against the Comparator Method.
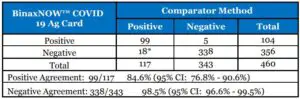

*14 of the discrepant samples had high Ct values (>33) when tested by the comparator method.The following data is provided for informational purposes:The performance of BinaxNOW™ COVID-19 Ag Card with positive results stratified by the comparator method cycle threshold (Ct) counts were collected and assessed to better understandthe correlation of assay performance to the cycle threshold, estimating the viral titer present in the clinical sample. As presented in the table below, the positive agreement of the BinaxNOW™COVID-19 Ag Card is higher with samples of a Ct count <33.
BinaxNOW™ COVID-19 Ag Card Performance against the Comparator Method – by Cycle Threshold Counts.


*In patients presenting within seven (7) days of symptom onset, BinaxNOW COVID-19 Ag Card achieved 95.6% (86/90) positive percent agreement for samples with Ct < 33
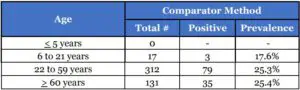
Patient Demographics
Patient demographics (gender and age) are available for the 460 samples used in the analysis of patients with symptom onset within the previous seven (7) days. The table below shows the positive results broken down by age of the patient:

Patient demographics, time elapsed since onset of symptoms for all patients enrolled, are presented in the table below. Positive results broken down by days since symptom onset:


A cohort of patients who presented with symptom onset greater than seven days were enrolled in the clinical study (n = 161). The positive agreement in patients with symptoms greater than sevendays was 60% (30/50) and negative agreement was 98% (109/111). Therefore, negative results in patients with symptom onset greater than seven days should be interpreted with caution, as the sensitivity of the assay decreases over time.
ANALYTICAL PERFORMANCELimit of Detection (Analytical Sensitivity)
BinaxNOW™ COVID-19 Ag Card limit of detection (LOD) was determined by evaluating different concentrations of heat inactivated SARS-CoV-2 virus. Presumed negative natural nasal swab specimens were eluted in PBS. Swab eluates were combined and mixed thoroughly to create a clinical matrix pool to be used as the diluent. Inactivated SARS-CoV-2 virus was diluted in thisnatural nasal swab matrix pool to generate virus dilutions for testing.Contrived nasal swab samples were prepared by absorbing 20 microliters of each virus dilution onto the swab. The contrived swab samples were tested according to the test procedure.The LOD was determined as the lowest virus concentration that was detected ≥ 95% of the time (i.e., concentration at which at least 19 out of 20 replicates tested positive).The BinaxNOW™ COVID-19 Ag Card LOD in natural nasal swab matrix was confirmed as 140.6 TCID50/mL.
Limit of Detection (LoD) Study Results


Cross Reactivity (Analytical Specificity) and Microbial Interference
Cross reactivity and potential interference of BinaxNOW™ COVID-19 Ag Card was evaluated by testing 37 commensal and pathogenic microorganisms (8 bacteria, 14 viruses, 1 yeast and pooled human nasal wash) that may be present in the nasal cavity. Each of the organism, viruses, and yeast were tested in triplicate in the absence or presence of heat inactivated SARS-CoV-2 virus(45 TCID50/swab). No cross-reactivity or interference was seen with the following microorganisms when tested at the concentration presented in the table below.
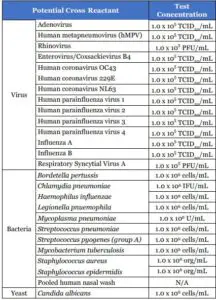

To estimate the likelihood of cross-reactivity with SARS-CoV-2 virus in the presence of organisms that were not available for wet testing, In silico analysis using the Basic Local Alignment SearchTool (BLAST) managed by the National Center for Biotechnology information (NCBI) was used to assess the degree of protein sequence homology.
- For P. jirovecii one area of sequence similarity shows 45% homology across 18% of the sequence, making cross-reactivity in the BinaxNOW™ COVID-19 Ag Card highly unlikely.
- No protein sequence homology was found between M. tuberculosis, and thus homologybased cross-reactivity can be ruled out.
- The comparison between SARS-CoV-2 nucleocapsid protein, MERS-CoV and human coronavirus HKU1 revealed that cross-reactivity cannot be ruled out. Homology for KHU1 and MERS-CoV is relatively low, at 37.8% across 95% of the sequence and 57.14% across 87% of the sequence, respectively
High Dose Hook Effect
No high dose hook effect was observed when tested with up to a concentration of 1.6 x 105 TCID50/mL of heat inactivated SARS-CoV-2 virus with the BinaxNOW™ COVID-19 Ag Card.
Endogenous Interfering Substances
The following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal cavity or nasopharynx, were evaluated with the BinaxNOW™ COVID19 Ag Card at the concentrations listed below and were found not to affect test performance.


1-Testing demonstrated false negative results at concentrations of 5 mg/mL (0.5% w/v). Standard dose of nasal ointment: 20 mg (2% w/w) of mupirocin in single-use 1-gram tubes.
ORDERING AND CONTACT INFORMATION
Reorder Numbers:195-000: BinaxNOW™ COVID-19 Ag Card (40 Tests)195-080: BinaxNOW™ COVID-19 Ag Control Swab Kit
US +1 877 441 7440Technical Support Advice LineFurther information can be obtained from your distributor, or by contacting Technical Support on:
US+ 1 800 257 9525 [email protected]
Abbott Diagnostics Scarborough, Inc. 10 Southgate Road Scarborough, Maine 04074 USA www.abbott.com/poct
FAQS
The BinaxNOW COVID-19 Antigen Self Test does not currently pair with the NAVICA app. The BinaxNOW COVID-19 Home Test can provide a sharable digital COVID-19 result, through collaboration with eMed. The BinaxNOW COVID-19 Antigen Self Test is the identical test card to the professional test but can be purchased at retail locations without a prescription. There’s also no need for a healthcare professional or online proctor to administer the swab or interpret results. Please let us know if there is anything else we can help you with.
This is an antigen test, not as accurate as a PCR molecular test. Depends on the viral load in your nasal passages. If positive you are likely infected with Covid. If negative and have symptoms test again and get a PCR test.
No. The BinaxNOW COVID-19 Ag Card is for testing only patients who are suspected of having SARS by their healthcare provider.
Yes. The BinaxNOW COVID-19 Ag Card is for testing nasal swabs from contacts of suspected SARS patients.
If your patient has a negative result on the BinaxNOW COVID-19 Ag Card and remains ill, contact your local or state health department immediately.
If your patient has a positive result on the BinaxNOW COVID-19 Ag Card and is not sick, contact your local or state health department immediately.
If your patient has a positive result on the BinaxNOW COVID-19 Ag Card and is not available for further testing, contact your local or state health department immediately.
The sample must be tested as soon as possible after collection. Do not store samples longer than 24 hours at 2 to 8 degrees Celsius (36 to 46 degrees Fahrenheit). Do not freeze samples. After 24 hours, samples should be discarded.
Abbott is intently monitoring the mutations of COVID so we can ensure our tests can detect them, as we do with many viruses. We have conducted a thorough analysis of known variants that we’ve been able to study, and we are confident that our tests remain effective at identifying these strains. It is highly likely that future COVID-19 strains will remain detectable because our tests – and most other COVID-19 tests – look for parts of the virus that are crucial for the virus’ survival and therefore less subject to mutation. Please feel free to call 1-833-637-1594 if you need further assistance.
No, this is an antigen test that only detects an active infection. Antibody tests can detect past infections. Please let us know if there is anything else we can help you with.
Based on the interim results of a clinical study where the BinaxNOW™ COVID-19 Antigen Self Test was compared to an FDA authorized high sensitivity SARS-CoV-2 test, BinaxNOW COVID-19 Antigen Self Test correctly identified 84.6% of positive specimens and 98.5% of negative specimens.
Based on the interim results of a clinical study where the BinaxNOW™ COVID-19 Antigen Self Test was compared to an FDA authorized high sensitivity SARS-CoV-2 test, BinaxNOW COVID-19 Antigen Self Test correctly identified 84.6% of positive specimens and 98.5% of negative specimens.
That’s up to individual insurance companies to determine. Some cover other Over-the-Counter products so individuals would need to reach out to their provider to determine coverage.
We are producing millions of rapid tests per month here in the U.S. and, as we have been throughout the entire pandemic, are committed to making rapid tests available to more people at affordable prices and convenient places.
The QR code on a BinaxNOW COVID-19 test card is used by NAVICA, Abbott’s COVID-19 digital testing system. NAVICA can be used to easily capture, manage and share self reported results from the BinaxNOW COVID-19 Antigen Self Test. It is available at no cost on the App Store or Google Play.
VIDEO
BinaxNOW Covid-19 Antigen Test Instructions – BinaxNOW Covid-19 Antigen Test Instructions –


[xyz-ips snippet=”download-snippet”]


