BinaxNOW™COVID-19 Ag CARD
For use with nasal swab specimensFor in vitro Use Only
INTENDED USE
The BinaxNOW™ COVID-19 Ag Card is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARSCoV-2 in direct nasal swabs from individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. The BinaxNOW COVID-19 Ag Card does not differentiate between SARSCoV and SARS-CoV-2.Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in nasal swabs during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results from patients with symptom onset beyond seven days, should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.The BinaxNOW COVID-19 Ag Card is intended for use by medicalprofessionals or trained operators who are proficient in performing rapid lateral flow tests. BinaxNOW COVID-19 Ag Card is only for use under the Food and Drug Administration’s Emergency Use Authorization.
SUMMARY and EXPLANATION of the TEST
Coronaviruses are a large family of viruses which may cause illness in animals or humans. SARS-CoV-2 is an enveloped, single-stranded RNA virus of the β genus. The virus can cause mild to severe respiratory illness and has spread globally, including the United States.BinaxNOW COVID-19 Ag Card is a rapid lateral flow immunoassay for thequalitative detection and diagnosis of SARS-CoV-2 directly from nasal swabs, without viral transport media.The BinaxNOW COVID-19 Ag Card kit contains all components required tocarry out an assay for SARS-CoV-2.
PRINCIPLES of the PROCEDURE
The BinaxNOW COVID-19 Ag Card is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab specimens. SARS-CoV-2 specific antibodies and a control antibody are immobilized onto a membrane support as two distinct lines and combined with other reagents/pads to construct a test strip. This test strip and a well to hold the swab specimen are mounted on opposite sides of a cardboard, book-shaped hinged test card.To perform the test, a nasal swab specimen is collected from the patient, 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab well. The patient sample is inserted into the test card through the bottom hole of the swab well, and firmly pushed upwards until the swab tip is visible through the top hole. The swab is rotated 3 times clockwise and the card is closed, bringing the extracted sample into contact with the test strip. Test results are interpreted visually at 15 minutes based on the presence or absence of visually detectable pink/purple colored lines. Results should not be read after 30 minutes.
REAGENTS and MATERIALS
Materials Provided
Test Cards (40): A cardboard, book-shaped hinged test card containing thetest stripExtraction Reagent (1): Bottle containing 10 mL of extraction reagentNasal Swabs (40): Sterile swabs for use with BinaxNOW COVID-19 Ag Card testPositive Control Swab (1): Non-infectious recombinant SARS-CoV-2nucleocapsid antigen dried onto a swabNegative Control Swab: The use of a sterile patient swab ensures appropriatenegative results are obtainedProduct Insert (1)Procedure Card (1)Materials Required but not ProvidedClock, timer or stopwatchMaterials Available as an Optional AccessorySwab Transport Tube Accessory Pack
PRECAUTIONS
- For in vitro diagnostic use.
- This test has not been FDA cleared or approved; this test has been authorized by FDA under an EUA for use by laboratories certified underthe Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42U.S.C. §263a, to perform moderate, high, or waived complexity tests and at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
- Federal Law restricts this device to sale by or on the order of a licensedpractitioner (US only).
- This test has been authorized only for the detection of SARS-CoV-2antigen, not for any other viruses or pathogens.
- This test is only authorized for the duration of the declaration thatcircumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
- Laboratories within the United States and its territories are required toreport all positive results to the appropriate public health laboratories.
- Treat all specimens as potentially infectious. Follow universal precautions when handling samples, this kit and its contents.
- Proper sample collection, storage and transport are essential for correctresults.
- Leave test card sealed in its foil pouch until just before use. Do not use if pouch is damaged or open.
- Do not use kit past its expiration date.
- Do not mix components from different kit lots.
- Do not reuse the used test card.
- Inadequate or inappropriate sample collection, storage, and transport may yield false test results.
- Do not store specimens in viral transport media for specimen storage.
- All components of this kit should be discarded as Biohazard waste according to Federal, State and local regulatory requirements.
- Solutions used to make the positive control swab are non-infectious .However, patient samples, controls, and test cards should be handled asthough they could transmit disease. Observe established precautions against microbial hazards during use and disposal.
- Wear appropriate personal protection equipment and gloves when running each test and handling patient specimens. Change gloves between handling of specimens suspected of COVID-19.
- INVALID RESULTS can occur when an insufficient volume of extraction reagent is added to the test card. To ensure delivery of adequate volume, hold vial vertically, 1/2 inch above the swab well, and add drops slowly.
- False Negative results can occur if the sample swab is not rotated (twirled) prior to closing the card.Swabs in the kit are approved for use with BinaxNOW COVID-19 Ag Card. Do not use other swabs.
- The Extraction Reagent packaged in this kit contains saline, detergents and preservatives that will inactivate cells and virus particles. Samples eluted in this solution are not suitable for culture.
- Do not store the swab after specimen collection in the original paperpackaging, if storage is needed use a plastic tube with cap.
STORAGE and STABILITY
Store kit at 2-30°C. The BinaxNOW COVID-19 Ag Card kit is stable until the expiration date marked on the outer packaging and containers. Ensure all test components are at room temperature before use.
QUALITY CONTROL
BinaxNOW COVID-19 Ag Card has built-in procedural controls. For dailyquality control, Abbott suggests that you record these controls for each test run. Procedural Controls:A. The pink-to-purple line at the “Control” position is an internal procedural control. If the test flows and the reagents work, this line will always appear.B. The clearing of background color from the result window is a negativebackground control. The background color in the window should be lightpink to white within 15 minutes. Background color should not hinderreading of the test. External Positive and Negative Controls:Good laboratory practice suggests the use of positive and negative controls to ensure that test reagents are working and that the test is correctly performed. BinaxNOW COVID-19 Ag Card kits contain a Positive Control Swab and Sterile Swabs that can be used as a Negative Control Swab. These swabs will monitor the entire assay. Test these swabs once with each new shipment received and once for each untrained operator. Further controls may be tested in order to conform with local, state and/or federal regulations, accrediting groups, or your lab’s standard Quality Control procedures.If the correct control results are not obtained, do not perform patient tests orreport patient results. Contact Technical Support during normal business hours before testing patient specimens.
SPECIMEN COLLECTION and HANDLING
Test specimens immediately after collection for optimal test performance.Inadequate specimen collection or improper sample handling/storage/transport may yield erroneous results. Refer to the CDC Interim Guidelinesfor Collecting, Handling, and Testing Clinical Specimens from Persons forCoronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.htmlNasal SwabOnly the swab provided in the kit is to be used for nasal swab collection.To collect a nasal swab sample, carefully insert the swab into the nostril exhibiting the most visible drainage, or the nostril that is most congested if drainage is not visible. Using gentle rotation, push the swab until resistance is met at the level of the turbinates (less than one inch into the nostril). Rotate the swab 5 times or more against the nasal wall then slowly remove from the nostril. Using the same swab, repeat sample collection in the other nostril.
SPECIMEN TRANSPORT and STORAGE
Do not return the nasal swab to the original paper packaging.For best performance, direct nasal swabs should be tested as soon as possibleafter collection. If immediate testing is not possible, and to maintain bestperformance and avoid possible contamination, it is highly recommendedthe nasal swab is placed in a clean, unused plastic tube labeled with patientinformation, preserving sample integrity, and capped tightly at roomtemperature (15-30°C) for up to (1) hour prior to testing. Ensure the swab fits securely within the tube and the cap is tightly closed. If greater than 1 hour delay occurs, dispose of sample. A new sample must be collected for testing.
TEST PROCEDURE
Procedure for Patient SpecimensOpen the test card just prior to use, lay it flat, and perform assay as follows.The test card must be flat when performing testing, do not perform testing with the test card in any other position.
- Hold Extraction Reagent bottle vertically. Hovering 1/2 inch above the TOP HOLE, slowly add 6 DROPS to the TOP HOLEof the swab well. DO NOT touch the card with the dropper tip while dispensing.
- Insert sample into BOTTOM HOLE and firmly push upwards so that the swab tip is visible in the TOP HOLE.

- Rotate (twirl) swab shaft 3 timesCLOCKWISE (to the right). Do notremove swab.
 Note: False negative results can occur if the sample swab is not rotated (twirled) prior to closing the card.
Note: False negative results can occur if the sample swab is not rotated (twirled) prior to closing the card. - Peel off adhesive liner from the right edge of the test card. Close and securely seal the card. Read result in the window 15 minutes after closing the card. In order to ensure proper test performance, it is important to read the result promptly at 15 minutes, and not before. Results should not be read after 30 minutes.
 Note: When reading test results, tilt the card to reduce glare on the result window if necessary. Individuals with color-impaired vision may not be able to adequately interpret test results.
Note: When reading test results, tilt the card to reduce glare on the result window if necessary. Individuals with color-impaired vision may not be able to adequately interpret test results.
Procedure for BinaxNOW™ Swab Controls
Open the test card just prior to use, lay it flat, and perform assay as follows.
- Hold Extraction Reagent bottle vertically Hovering 1/2 inch above the TOP HOLE, slowly add 8 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.

- Follow Steps 2 – 4 of the Test Procedure for Patient Specimens.
RESULT INTERPRETATION
Note: In an untested BinaxNOW COVID-19 Ag Card there will be a blue line present at the Control Line position. In a valid, tested device, the blue line washes away and a pink/purple line appears, confirming that the sample has flowed through the test strip and the reagents are working. If the blue line is not present at the Control Line position prior to running the test, do not use and discard the test card.
Negative
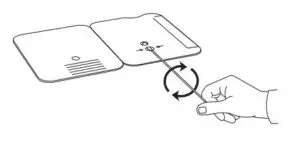
A negative specimen will give a single pink/purple colored Control Line inthe top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected.
Positive

A positive specimen will give two pink/ purple colored lines. This means that COVID-19 antigen was detected. Specimens with low levels of antigen may give a faint Sample Line. Any visible pink/ purple colored line is positive.
Invalid

If no lines are seen, if just the Sample Line is seen, or the Blue Control Lineremains blue, the assay is invalid. Invalid tests should be repeated.
LIMITATIONS
- This test detects both viable (live) and non-viable, SARS-CoV and SARSCoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- The performance of the BinaxNOW COVID-19 Ag Card was evaluatedusing the procedures provided in this product insert only. Modifications to these procedures may alter the performance of the test.
- False negative results may occur if a specimen is improperly collected, transported, or handled.
- False results may occur if specimens are tested past 1 hour of collection.Specimens should be tested as quickly as possible after specimen collection.
- False negative results may occur if inadequate extraction buffer is used (e.g., <6 drops).
- False negative results may occur if specimen swabs are not twirled within the test card.
- False negative results may occur if swabs are stored in their paper sheath after specimen collection.
- Positive test results do not rule out co-infections with other pathogens.
- Positive test results do not differentiate between SARS-CoV and SARSCoV-2.
- Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
- The presence of mupirocin may interfere with the BinaxNOW COVID 19 Ag test and may cause false negative results.
- Negative results, from patients with symptom onset beyond seven days, should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed.
- If the differentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local public health departments, is required.
The BinaxNOW COVID-19 Ag Card Letter of Authorization, along with theauthorized Fact Sheet for Healthcare Providers, the authorized Fact Sheet forPatients, and authorized labeling are available on the FDA website: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-useauthorizations-medical-devices/vitro-diagnostics-euas.However, to assist clinical laboratories using the BinaxNOW COVID-19 AgCard, the relevant Conditions of Authorization are listed below:
- Authorized laboratories1 using your product will include with test resultreports, all authorized Fact Sheets. Under exigent circumstances, otherappropriate methods for disseminating these Fact Sheets may be used, which may include mass media.
- Authorized laboratories using your product will use your product as outlined in the “BinaxNOW COVID-19 Ag Card” Instructions for Use. Deviations from the authorized procedures, including the authorized instruments, authorized clinical specimen types, authorized control materials, authorized other ancillary reagents and authorized materials required to use your product are not permitted.
- Authorized laboratories that receive your product will notify the relevant public health authorities of their intent to run your product prior to initiating testing.
- Authorized laboratories using your product will have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
- Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: [email protected]) and Abbott DiagnosticsScarborough, Inc. (via email: , or via phone by contacting Abbott Diagnostics Scarborough, Inc. Technical Service at 1-800-257- 9525 any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of your product of which they become aware.
- All operators using your product must be appropriately trained in performing and interpreting the results of your product, use appropriate personal protective equipment when handling this kit, and use your product in accordance with the authorized labeling
- Abbott Diagnostics Scarborough, Inc., authorized distributors, and authorized laboratories and patient care settings using your product will ensure that any records associated with this EUA are maintained until otherwise notified by FDA. Such records will be made available to FDA for inspection upon request.
1 The letter of authorization refers to, “Laboratories certified under the Clinical
Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a,that meet the requirements to perform high, moderate, or waived complexitytests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.” as “authorized laboratories.”
PERFORMANCE CHARACTERISTICS
CLINICAL PERFORMANCE
Clinical performance characteristics of BinaxNOW COVID-19 Ag Cardwas evaluated in a multi-site prospective study in the U.S in which patientswere sequentially enrolled and tested. A total of seven (7) investigationalsites throughout the U.S. participated in the study. Testing was performedby operators with no laboratory experience and who are representative ofthe intended users at CLIA waived testing sites. In this study testing wasconducted by thirty-two (32) intended users. No training on the use of thetest was provided to the operators. To be enrolled in the study, patients had tobe presenting at the participating study centers with suspected COVID-19.Patients who presented within 7 days of symptom onset were included in theinitial primary analysis, as only seven asymptomatic patients were enrolled.Of the seven asymptomatic patients, only two patients were positive forSARS-CoV-2. Two nasal swabs were collected from patients and tested using the BinaxNOW COVID-19 Ag Card at all study sites. An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-CoV-2 was utilized as the comparator method for this study. At all sites, one nasal swab was tested directly in the BinaxNOW COVID-19 Ag Card test according to product instructions and the other swab was eluted in viral transport media (VTM). Swabs were randomly assigned to testing with the BinaxNOW or RT-PCR testing and were tested by minimally trained operators who were blinded to the RT-PCR test result. All sites shipped the VTM sample to a central testing laboratory for RT-PCR. External control testing, using BinaxNOW COVID-19 Ag Card Positive and Negative Controls, was performed prior to sample testing each day, at all study sites. The performance of BinaxNOW COVID-19 Ag Card was established with 102 nasal swabs collected from individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW™ COVID-19 Ag Card Performance within 7 days of symptom onset against the Comparator Method.

Patient Demographics
Patient demographics (gender, age, time elapsed since onset of symptoms) are available for the 102 samples used in the analysis. The table below shows the positive results broken down by age of the patient:

Positive results broken down by days since symptom onset:

The following data is provided for informational purposes:The performance of BinaxNOW COVID-19 Ag Card with positive results stratified by the comparator method cycle threshold (Ct) counts were collected and assessed to better understand the correlation of assay performance to the cycle threshold, estimating the viral titer present in the clinical sample. As presented in the table below, the positive agreement of the BinaxNOW COVID-19 Ag Card is higher with samples of a Ct count <33.
BinaxNOW™ COVID-19 Ag Card Performance against the Comparator Method – by Cycle Threshold Counts.

A limited cohort of patients who presented with symptom onset greater thanseven days were enrolled in the clinical study (n = 28). Although the sample size was relatively small, the positive agreement in this cohort was 75% (9/12) and negative agreement was 92% (11/12). Therefore, negative results in patients with symptom onset greater than seven days should be treated as presumptive and confirmed with a molecular assay if needed for clinical management.
ANALYTICAL PERFORMANCE:
Limit of Detection (Analytical Sensitivity)BinaxNOW COVID-19 Ag Card limit of detection (LOD) was determinedby evaluating different concentrations of heat inactivated SARS-CoV-2 virus. Presumed negative natural nasal swab specimens were eluted in PBS. Swab eluates were combined and mixed thoroughly to create a clinical matrix pool to be used as the diluent. Inactivated SARS-CoV-2 virus was diluted in this natural nasal swab matrix pool to generate virus dilutions for testing. Contrived nasal swab samples were prepared by absorbing 20 microliters of each virus dilution onto the swab. The contrived swab samples were tested according to the test procedure.The LOD was determined as the lowest virus concentration that was detected≥ 95% of the time (i.e., concentration at which at least 19 out of 20 replicatestested positive).The BinaxNOW COVID-19 Ag Card LOD in natural nasal swab matrix wasconfirmed as 22.5 TCID50/swab.Limit of Detection (LoD) Study Results.

Cross Reactivity (Analytical Specificity) and Microbial Interference
Cross reactivity and potential interference of BinaxNOW COVID-19 AgCard was evaluated by testing 37 commensal and pathogenic microorganisms (8 bacteria, 14 viruses, 1 yeast and pooled human nasal wash) that may be present in the nasal cavity. Each of the organism, viruses, and yeast were tested in triplicate in the absence or presence of heat inactivated SARS-CoV-2 virus (45 TCID50/swab). No cross-reactivity or interference was seen with the following microorganisms when tested at the concentration presented in the table below.


To estimate the likelihood of cross-reactivity with SARS-CoV-2 virus in thepresence of organisms that were not available for wet testing, In silico analysis using the Basic Local Alignment Search Tool (BLAST) managed by the National Center for Biotechnology Information (NCBI) was used to assess the degree of protein sequence homology.
- For P. jirovecii one area of sequence similarity shows 45% homologyacross 18% of the sequence, making cross-reactivity in the BinaxNOW COVID-19 Ag Card highly unlikely.
- No protein sequence homology was found between M. tuberculosis, and thus homology-based cross-reactivity can be ruled out.
- The comparison between SARS-CoV-2 nucleocapsid protein, MERS CoV and human coronavirus HKU1 revealed that cross-reactivity cannot be ruled out. Homology for KHU1 and MERS-CoV is relatively low, at 37.8% across 95% of the sequence and 57.14% across 87% of the sequence, respectively.
High Dose Hook Effect
No high dose hook effect was observed when tested with up to a concentration of 1.6 x 105 TCID50/mL of heat inactivated SARS-CoV-2 virus with the BinaxNOW COVID-19 Ag Card.
Endogenous Interfering Substances
The following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal cavity or nasopharynx, were evaluated with the BinaxNOW COVID-19 Ag Card at the concentrations listed below and were found not to affect test performance.

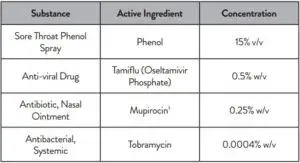
1 Testing demonstrated false negative results at concentrations of 5 mg/mL (0.5% w/v). Standard dose of nasal ointment: 20 mg (2% w/w) of mupirocin in single-use 1-gram tubes.
SYMBOLS

ORDERING and CONTACT INFORMATION
Reorder Numbers:195-000: BinaxNOW COVID-19 Ag Card (40 Tests)195-080: BinaxNOW COVID-19 Ag Control Swab Kit190-010: Swab Transport Tube Accessory PackUS +1 877 441 7440Technical Support Advice LineFurther information can be obtained from your distributor, or by contactingTechnical Support on:US+ 1 800 257 9525 [email protected]

BinaxNOW COVID-19 Ag Card Package Insert – BinaxNOW COVID-19 Ag Card Package Insert –
[xyz-ips snippet=”download-snippet”]

