
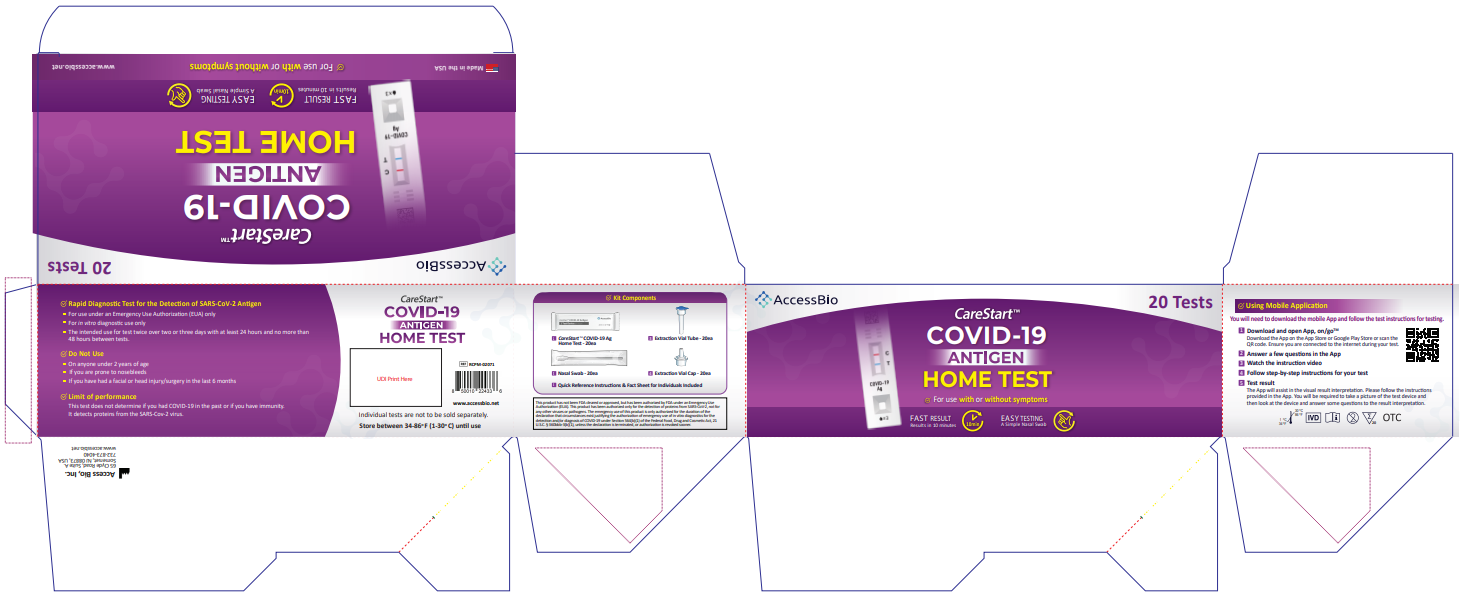
![]() RCPM – 00271, RCPM-00471, RCPM-02071
RCPM – 00271, RCPM-00471, RCPM-02071![]() COVID-19 Antigen Home TestUSER INSTRUCTIONS
COVID-19 Antigen Home TestUSER INSTRUCTIONS
You must follow the test directions carefully to get an accurate result. Call Technical Support at 1-888-898-1270 or visit accessbio.net to obtain the complete instructions for use. FOR USE UNDER EMERGENCY USE AUTHORIZATION (EUA) ONLY.![]() IMPORTANT: Swabbing the nostrils is critical for obtaining an accurate result.If you do not swab your nose, the device will produce a false negative result.
IMPORTANT: Swabbing the nostrils is critical for obtaining an accurate result.If you do not swab your nose, the device will produce a false negative result.
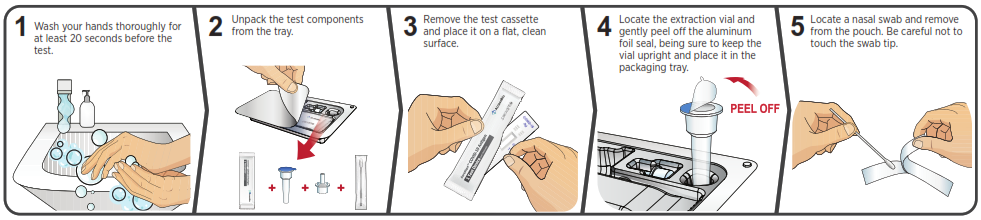

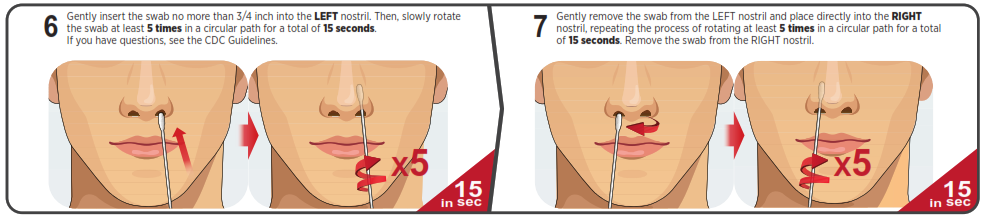

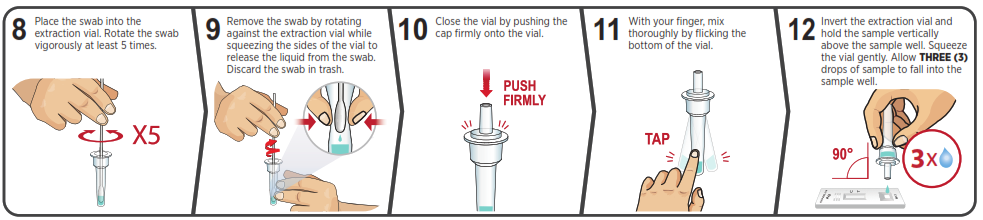

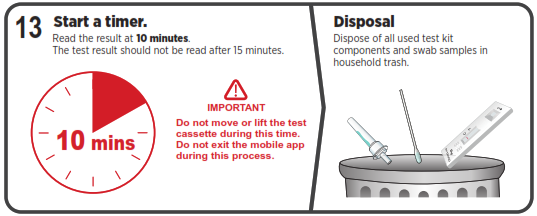

Using Mobile Application
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Scan the QR code to download


Please start the test follows the in-app self-paced, step-by-step test instructions.
- Download and open App, on/go TM Mobile ApplicationDownload the App on the App Store or Google Play Store. Ensure you are connected to the internet during your test.
- Answer a few questions in the App
- Watch the instruction video.
- Follow step-by-step instructions for your test.
- Test resultThe App will assist in the visual result interpretation. Please follow the instructions provided in the App. You will be required to take a picture of the test device and then look at the device and answer some questions to the result interpretation.
Results Interpretation![]()
![]()
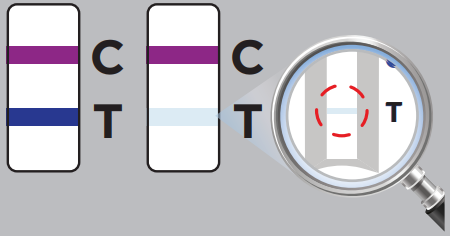
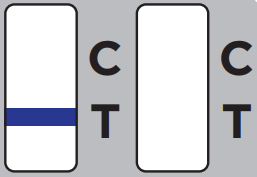
The test results will be interpreted by visual reading following the in-app interpretation instructions or provided Quick Reference Instructions.NOTE: The test results should be read by visual and interpreted at 10 minutes after the sample application and the reading and interpretation of the results should not exceed 15 minutes as it may yield inaccurate results.![]()
![]()

![]()
![]()
![]()
A positive test result indicates that antigens from SARS-CoV-2 were detected, and the patient is very likely to be infected with the virus and presumed to be contagious. Test results should always be considered in the context of clinical observations and epidemiological data in making a final diagnosis and patient management decisions. You should self-isolate at home and avoid contact with others as per CDC recommendations to stop spreading the virus to others.
![]()
![]()
One purple-colored line only next to “C” indicates a negative result.


![]()
![]()

Intended Use
The CareStart™ COVID-19 Antigen Home Test is a rapid, lateral flow immunoassay intended for the qualitative detection of SARS-CoV-2 nucleocapsid protein antigens from individuals with or without symptoms or other epidemiological reasons to suspect aCOVID-19infection when test edt wi cover two or three days with at least 24 hours and not more than 48 hours between tests. This test is authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected anterior nasal swab samples from individuals aged 2 years or older.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. The antigen is generally detectable in anterior nasal swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses and the agent detected may not be the definite cause of disease. Individuals who test Positive with the CareStart™ COVID-19 Antigen Home Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary. Negative results should be treated as presumptive and confirmation with a molecular assay for patient management may be performed if necessary. Negative results do not rule out SARS-CoV-2 infection, and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19.
For serial testing programs, additional confirmatory testing with a molecular test for negative Results may be necessary, if there is a high likelihood of COVID-19, such as an individual with close contact with COVID-19 or ith suspected exposure to COVID-19 or in communities with a high prevalence of infection. Additional confirmatory testing with a molecular test for positive results may also be necessary if there is a low likelihood of COVID-19, such as in individuals without known exposures to COVID-19 or residing in communities with a low prevalence of infection.
Individuals who test negative and continue to experience COVID-like symptoms of fever, cough, and/or shortness of breath may still have SARS-CoV-2 infection and should seek follow-up care from their healthcare provider.
Individuals should provide all results obtained with their product to their healthcare provider for public health reporting. All healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LVID) Test Code Mapping for SARS-CoV-2 Tests provided by CDC. The CareStart™ COVID-19 Antigen ome Test is authorized for non-prescription self-use and/or, as applicable for an adult lay user testing another person aged 2 years or older in a non-laboratory setting.
The CareStart™ COVID-19 Antigen Home Test is only for use under the Food and Drug Administration’s Emergency Use Authorization.
Important Note
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
DO’s
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
DON’Ts
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Hazardous Ingredients for Liquid Reagent
The extraction solution in the vial contains potentially harmful chemicals (see table below). If the solution contacts the skin or eye, flush with copious amounts of water. If irritation persists, seek medical advice: https://www.poison.org/contact-us or 1-800-222-1222.
| Chemical Name | GHS Code for each Ingredient | Concentration |
| Triton X-100 | H315, skin irritation | 1.5% |
| N-Lauroylsarcosine sodium salt | H315, skin irritation | 0.15% |
Frequently Asked Questions
What is COVID-19?COVID-19 is caused by the SARS-CoV-2 virus which is a new virus in humans causing a contagious respiratory illness. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. Older adults and people of any age who have underlying medical conditions have a higher risk of severe illness from COVID-19. Serious outcomes of COVID-19 include hospitalization and death. The SARS-CoV-2 virus can be spread to others not just while one is sick, but even before a person shows signs or symptoms of being sick (e.g., fever, coughing, di˝culty breathing, etc.). A full list of symptoms of COVID-19 can be found at the following link: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
What are the symptoms of COVID-19?Many individuals with confirmed COVID-19 have developed fever and/or symptoms of acute respiratory illness (e.g., cough, dyspnea), although some individuals experience only mild symptoms or no symptoms at all. The current information available to characterize the spectrum of a clinical illness associated with COVID-19 suggests that, when present, symptoms include cough, shortness of breath or dyspnea, fever, chills, myalgias, headache, sore throat, or new loss of taste or smell, nausea or vomiting or diarrhea. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. Signs and symptoms may appear any time from 2 to 14 days after exposure to the virus, and the median time to symptom onset is approximately 5 days.
What is serial testing?Serial testing is when a single person is tested for COVID-19 more than once. Because antigen tests are less sensitive than other COVID-19 tests and false results may occur, repeated testing may identify more individuals with COVID-19 infection than a single test. By repeating testing, it may be possible to more quickly identify cases of COVID-19 infection and reduce the spread of infection. Additional testing with molecular COVID-19 test may be necessary, depending on your individual risk factors and test results. It is important that you work with your healthcare provider to help you understand the next steps you should take.
What are the known and potential risks and benefits of the test?Potential risks include:
- Possible discomfort or other complications can happen during sample collection.
- Possible incorrect test result (see below for more information). Potential benefits include:
- The results, along with other information, can help you and your healthcare provider make informed recommendations about your care.
- The results of this test may help limit the potential spread of COVID-19 to your family and others in your community.
What if I have a positive test result?If you have a positive test result, it is very likely that you have COVID-19 because proteins from the virus that causes COVID-19 were found in your sample. Therefore, it is also likely that you may be placed in isolation to avoid spreading the virus to others. There is a very small chance that this test can give a positive result that is wrong (a false-positive result). If you test positive with the CareStart™ COVID-19 Antigen Home Test you should self-isolate and seek follow-up care with your healthcare provider as additional testing may be necessary. Your healthcare provider as additional testing may be necessary. Your healthcare provider will work with you to determine how best to care for you based on your test result(s) along with your medical history, and your symptoms.
What if I have a negative test result?A negative test result means that proteins from the virus that causes COVID-19 were not found in your sample. It is possible for this test to give a negative result that is incorrect (false negative) in some people with COVID-19. This means you could possibly still have COVID-19 even though the test is negative. The amount of antigen in a sample may decrease the longer you have symptoms of infection. In symptomatic people, specimens collected after you have had symptoms for more than five days may be more likely to be negative compared to a molecular assay.If you test negative and continue to experience COVID-19 like symptoms of fever, cough, and/or shortness of breath you should seek follow-up care with your healthcare provider. For example, your healthcare provider may suggest you need another test to determine if you have contracted the virus causing COVID-19. If you are concerned bout your COVID-19 infection status after testing or think you may need follow-up testing, please contact your healthcare provider.
Explanation of Symbols
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()





![]()
![]()
![]()
![]()
![]()




References
Contact Us | Poison Control
Access Bio – A TRUSTED PARTNER IN GLOBAL HEALTH
Access Bio – A TRUSTED PARTNER IN GLOBAL HEALTH
Access Bio – A TRUSTED PARTNER IN GLOBAL HEALTH
Interim Guidelines for Clinical Specimens for COVID-19 | CDC
Protein BLAST: search protein databases using a protein query
Contact Us | Poison Control
[xyz-ips snippet=”download-snippet”]

