Atlas Scientific B07VDMNB92 Gen 2 Consumer Grade pH Probe Double Junction Silver User Manual

Measurements
Specifications
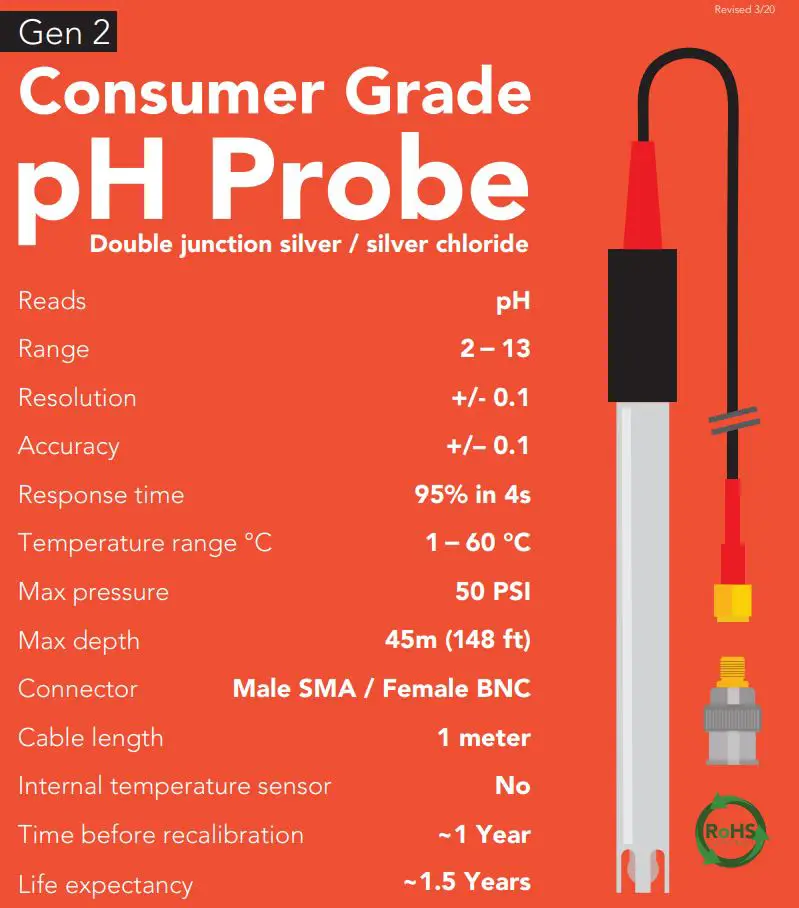
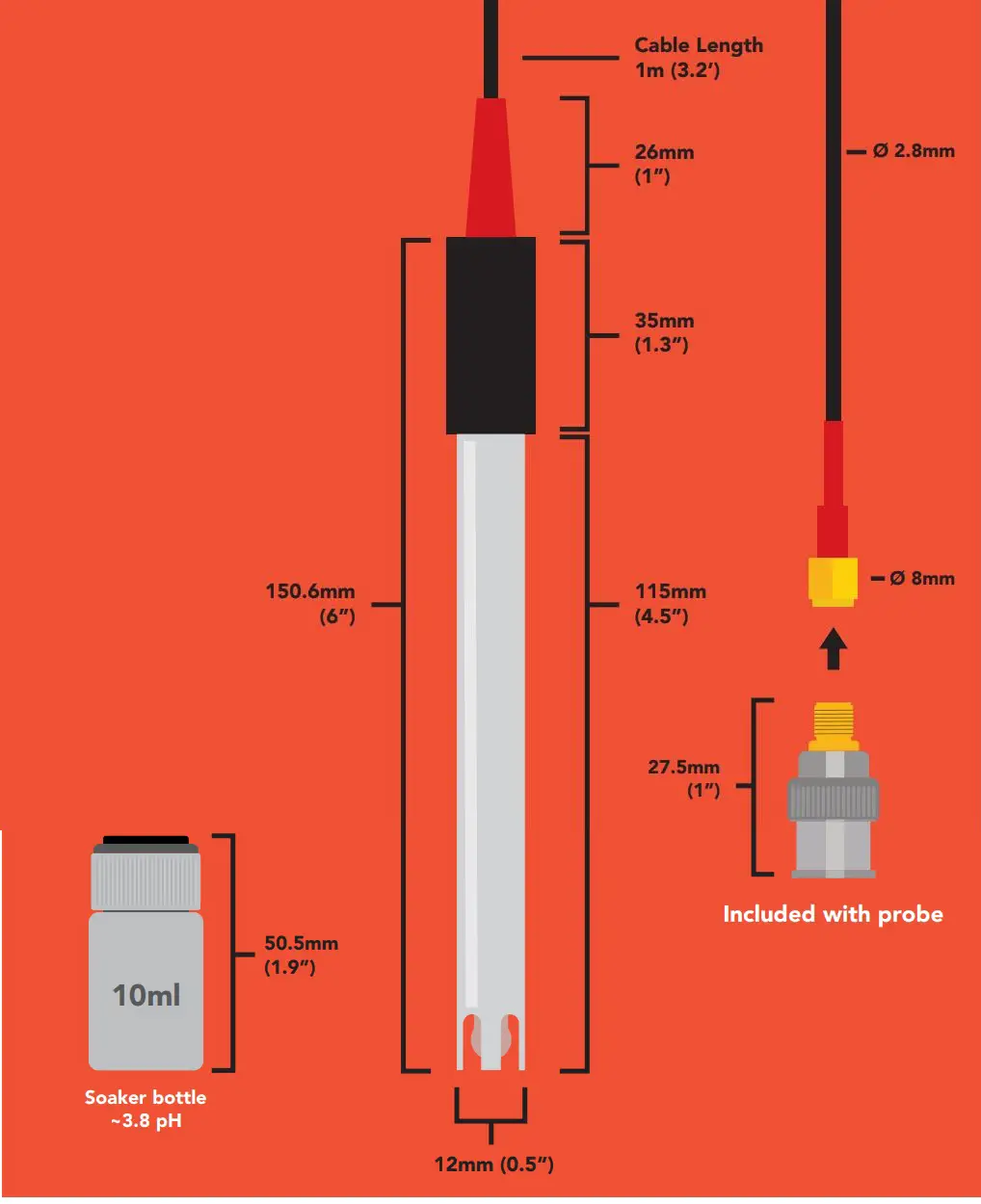
Reference electrode Silver / silver chlorideDouble junction YesMax depth 45m (148 ft.)Cable length 1 meterWeight 49 gramsSpeed of response 95% in 4 secondIsopotential point pH 7.00 (0 mV)Dimensions 12mm x 150.6mm (0.5″ x 6″)SMA connector FemaleSterilization Chemical onlyFood Safe YesIncluded with probe SMA to BNC adapter

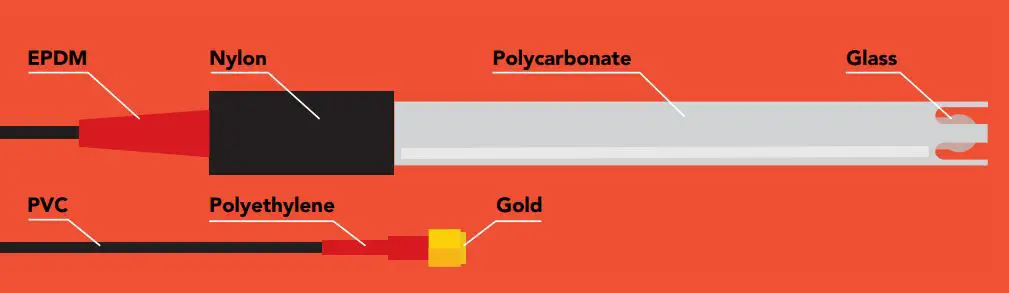
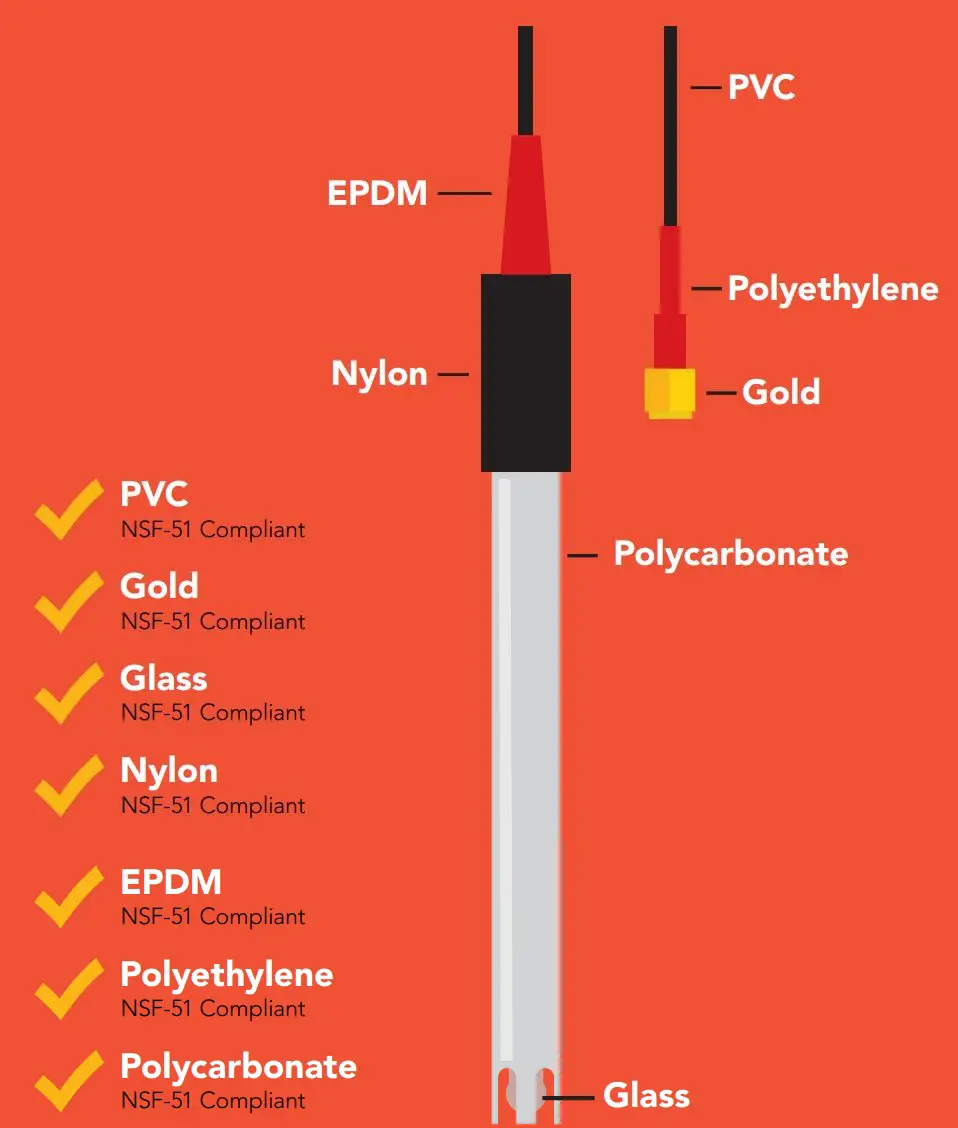
Materials
This pH probe can be fully submerged in fresh or salt water, up to the SMA connector indefinitely.
Typical applications
- Standard lab use
- Field use
- Soil
- Samples containing heavy metals
- Hydroponics / aquaponics
- Beer, wine, alcohol, and food production
NSF/ANSI 51 Compliant
Atlas Scientific LLC, hereby certifies that,Consumer Grade pH Probe Part # ENV-30-pH
Complies with NSF/ANSI Standard 51
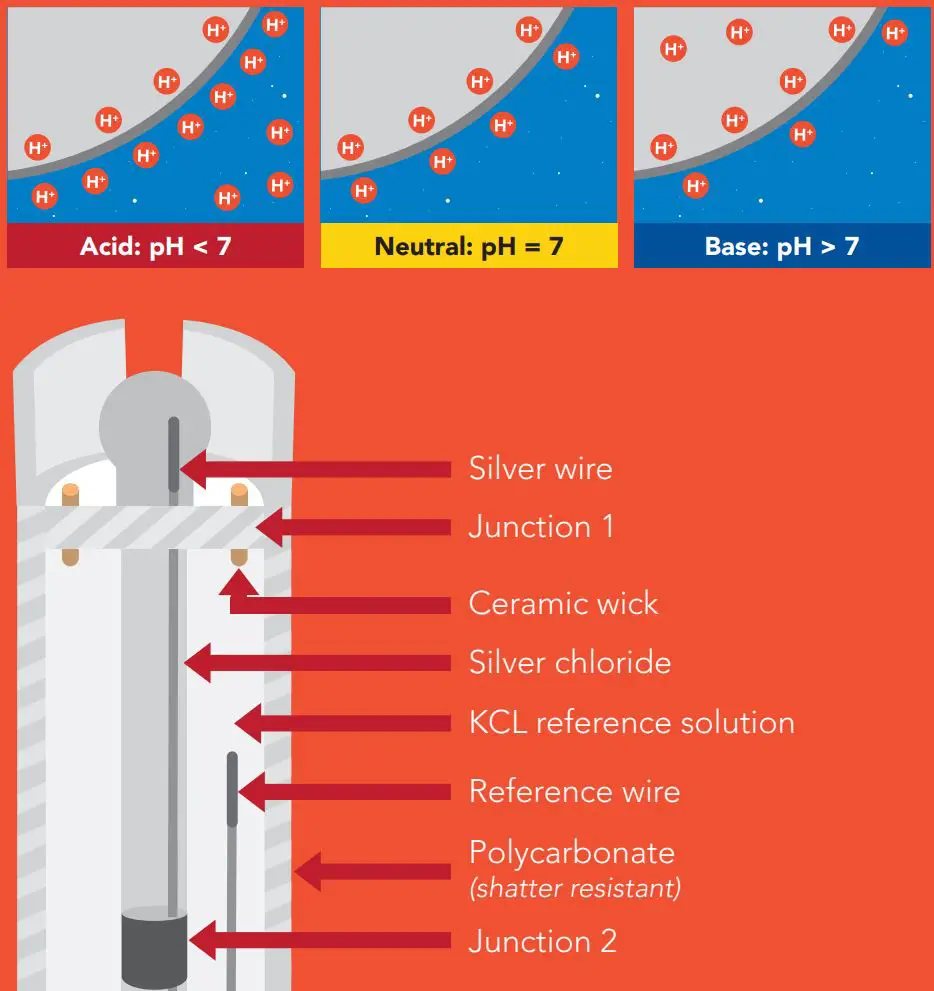
Operating principle
A pH (potential of Hydrogen) probe measures the hydrogen ion activity in a liquid. At the tip of a pH probe is a glass membrane. This glass membrane permits hydrogen ions from the liquid being measured to defuse into the outer layer of the glass, while larger ions remain in the solution. The difference in the concentration of hydrogen ions (outside the probe vs. inside the probe) creates a VERY small current. This current is proportional to the concentration of hydrogen ions in the liquid being measured.
A pH electrode is a passive device that detects a current generated from hydrogen ion activity. This current (which can be positive or negative) is very weak and cannot be detected with a multimeter, or an analog to digital converter. This weak electrical signal can easily be disrupted and care should be taken to only use proper connectors and cables. The current that is generated from the hydrogen ion activity is the reciprocal of that activity and can be predicted using this equation:
The current that is generated from the hydrogen ion activity is the reciprocal of that activity and can be predicted using this equation:

Where R is the ideal gas constant. T is the temperature in Kelvin. F is the Faraday constant.
Because a pH probe is a passive device it can pick up voltages that are transmitted through the solution being measured. This will result in incorrect readings and will slowly damage the pH probe over time. In this instance, proper isolation is required.
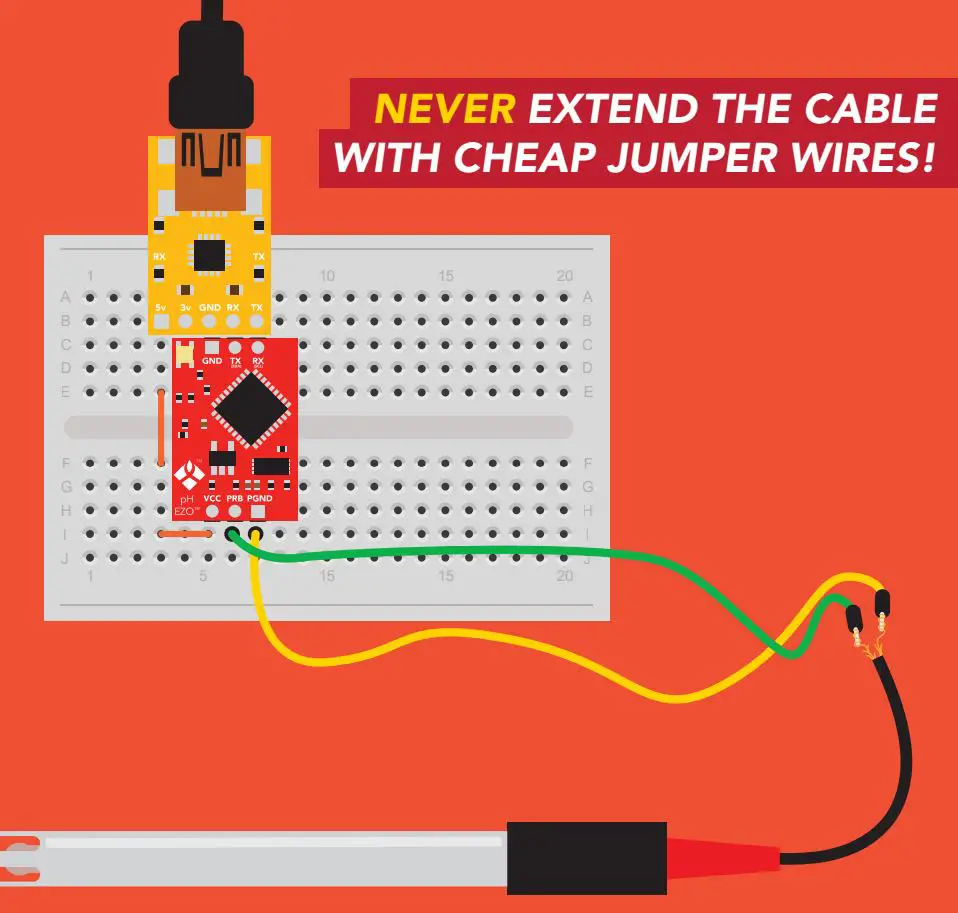
DO NOT CUT THE PROBE CABLE WITHOUT REFERRING TO THIS DOCUMENT!
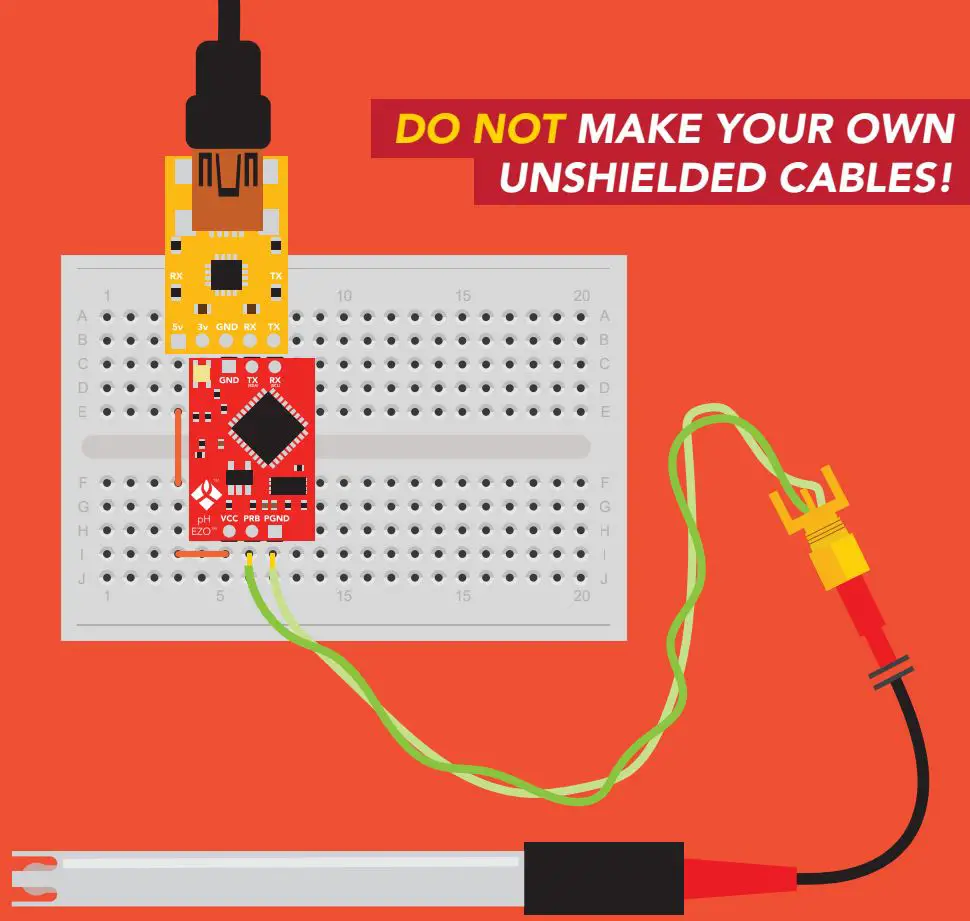
ONLY USE SHIELDED CABLES.
Extending the probe cable length
You can extend the cable to greater than 100 meters with no loss of signal. Atlas Scientific has tested up to 300 meters without a problem, however you run the risk of turning your pH probe into an antennae, picking up noise along the length of your cable.
If you want to extend your cable, we recommend that you use proper isolation, such as the Basic EZO TM Inline Voltage Isolator, or Tentacle Shield. Be sure to calibrate your probe with the extended cable.
Extending a probe cable can be easily done with our SMA Extension Cables. Simply connect the SMA end of the probe to the Extension cable, and you are all set.
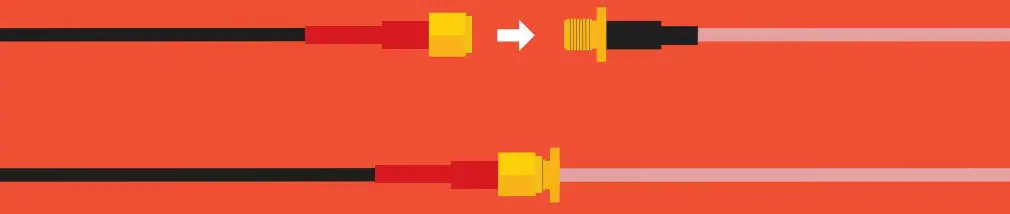
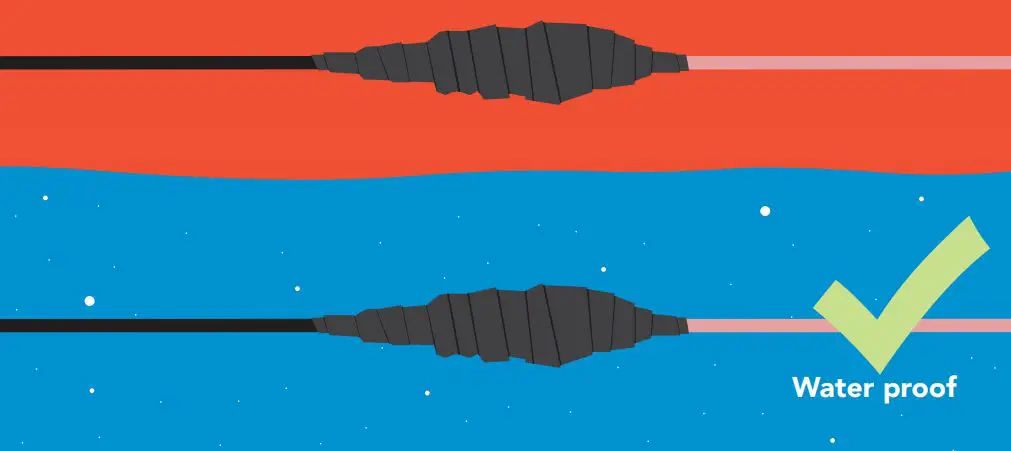
If you need to water proof a SMA connection, we highly recommend using a product like Coax-Seal to safely cover and prevent any water damage that may occur.
Helpful operating tips
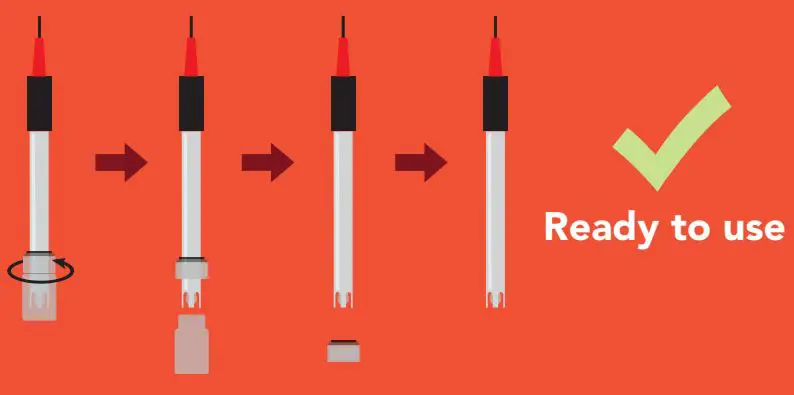
pH probes must stay wet and cannot be allowed to dry out, this is why every pH probe is shipped in a plastic soaker bottle containing pH probe storage solution. The probe should remain in the bottle until it is used. If the probe is used infrequently, the bottle and its solution should be saved and the probe stored inside.
- To remove the soaker bottle from the probe, hold the soaker bottle by the cap and turn the bottle until it separates from the cap; Then slide the cap off.Ready to use

- During shipment the air bubble in the probes stem may move into the bulb area. If bubbles are seen in the bulb area, hold the probe by its top cap and shake downward as done with a clinical thermometer. Bubbles

- Vigorously stir the probe in the sample, calibration solution, or rinse solution. This action will bring solution to the probes surface quicker and improve the speed of response.

Probe cleaning
Coating of the pH bulb can lead to erroneous readings including shortened span (slope). The type of coating will determine the cleaning technique. Soft coatings can be removed by vigorous stirring or by the use of a squirt bottle. Organic chemical, or hard coatings, should be chemically removed. A light bleach solution or even a 5 10% hydrochloric acid (HCl) soak for a few minutes, often removes many coatings. If cleaning does not restore performance, reconditioning may be tried. Do not use a brush or abrasive materials on the pH probe.

How often do you need to recalibrate a pH probe?
Because every use case is different, there is no set schedule for recalibration.
If you are using your probe in a fish tank, a hydroponic system or any environment that has generally weak levels of acids and bases you will only need to recalibrate your probe once per year for the first two years. After that every ~six months.
If you are using the pH probe in batch chemical manufacturing, industrial process, or in a solution that is known to have strong acids and bases, then calibration should be done monthly or in extreme cases after each batch.
Probe reconditioning
When reconditioning your pH probe is required due to aging, we recommend you use the Atlas Scientific pH probe reconditioning kit.

Copyright © Atlas Scientific LLC ![]()
[xyz-ips snippet=”download-snippet”]