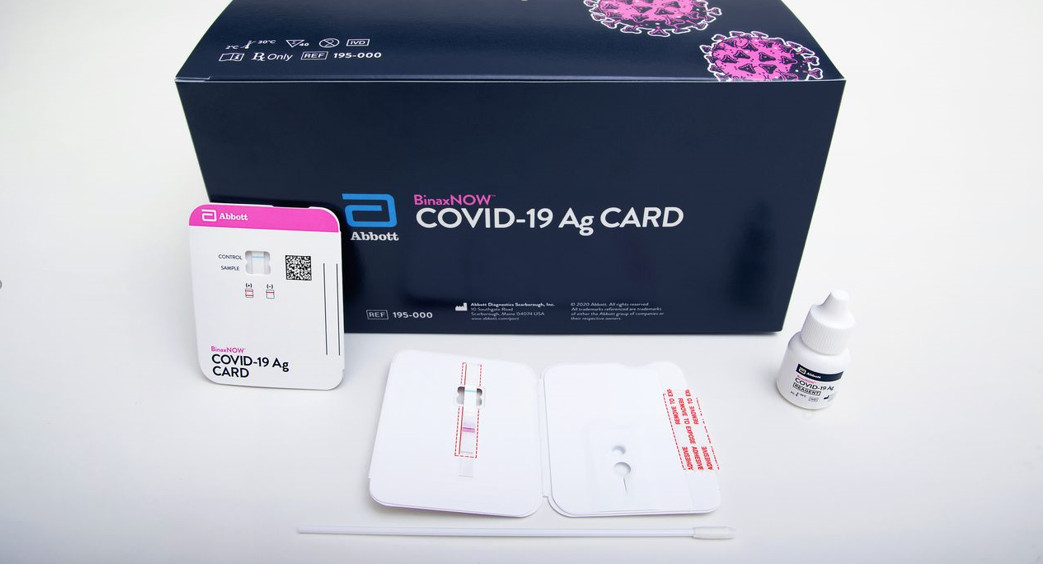
BinaxNOW TM COVID-19 Antigen Self TEST

Healthcare Provider Instructions for Use
For Use Under an Emergency Use Authorization (EUA) OnlyFor use with anterior nasal swab specimensFor in vitro Diagnostic Use Only
INTENDED USE
The BinaxNOW™ COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. This test is authorized for nonprescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 15 years or older or adult collected anterior nasal swab samples from individuals aged two years or older.
The BinaxNOW COVID-19 Antigen Self Test does not differentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen s are generally detectable in anterior nasal (nares) swabs during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with a past medical history and other diagnostic information is necessary to etermine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of the disease. Individuals who test positive with the BinaxNOW™ COVID-19 Antigen Self Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.
Negative results should be treated as presumptive, do not rule out SARS-CoV-2 infection, and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID19 and confirmed with a molecular assay, if necessary, for patient management. For serial testing programs, additional confirmatory testing ith a molecular test for negative results may be necessary, if there is a high likelihood of COVID-19, such as an individual with close contact with OVID-19 or with suspected exposure to COVID-19 or in communities with a high prevalence of infection. Additional confirmatory sting with a molecular test for positive results may also be necessary if there is a low likelihood of COVID-19, such as in individuals without known exposures to COVID-19 or residing in communities with a low prevalence of infection.
Individuals who test negative and continue to experience COVID-like symptoms of fever, cough, and/or shortness of breath may still have SARS-CoV-2 infection and should seek to follow-up care from their healthcare provider.
Individuals should report their test results through the NOVICA app and provide all results obtained with this product to their healthcare provider in order to receive appropriate medical care. All healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by CDC.
The BinaxNOW COVID-19 Antigen Self Test is intended for non-prescription self-use and/or, as applicable for an adult lay user testing another person aged 2 years or older in a non-laboratory setting. The BinaxNOW COVID-19 Antigen Self Test is only for use under the Food and Drug Administration’s Emergency Use Authorization.
SUMMARY AND EXPLANATION OF THE TEST
Coronaviruses are a large family of viruses that may cause illness in animals or humans. SARS-CoV-2 is an enveloped, single-stranded RNA virus of the β genus. The virus can cause mild to severe respiratory illness and has spread globally, including in the United States.
The BinaxNOW COVID-19 Antigen Self Test is a rapid lateral flow immunoassay for the qualitative detection of SARS-CoV-2 directly from anterior nasal swabs, without viral transport media. The BinaxNOW COVID-19 Antigen Self Test kit contains all components required to carry out an assay for SARS-CoV-2.
PRINCIPLES OF THE PROCEDURE
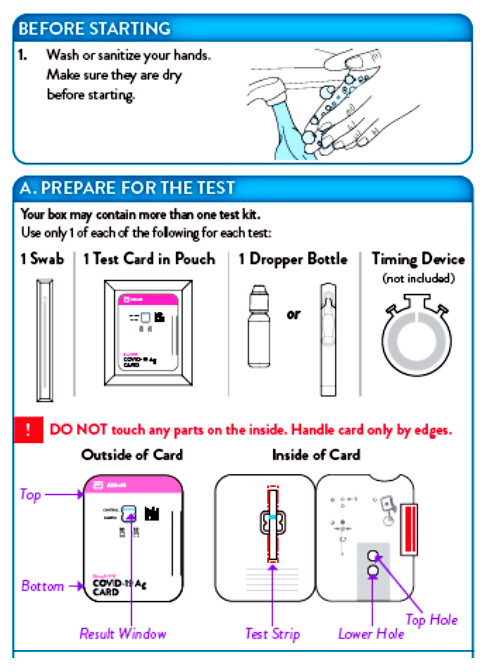
The BinaxNOW COVID-19 Antigen Self Test is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from direct anterior nasal swab specimens. SARS-CoV-2 specific antibodies and a control antibody are immobilized onto membrane support as two distinct lines and combined with other reagents/pads to construct a test strip. This test strip and a well to hold the swab specimen are mounted on opposite sides of a cardboard, book-shaped hinged test card.
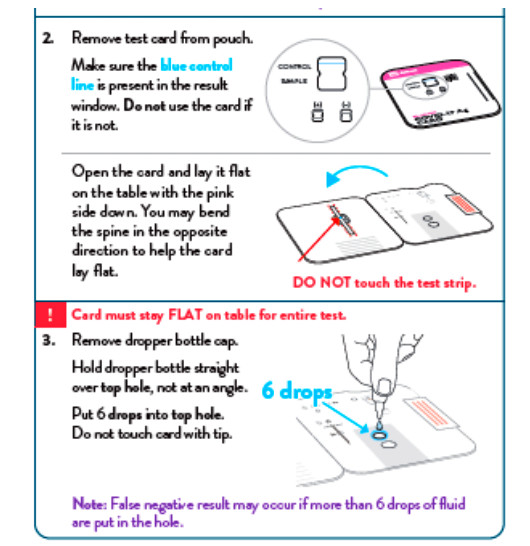
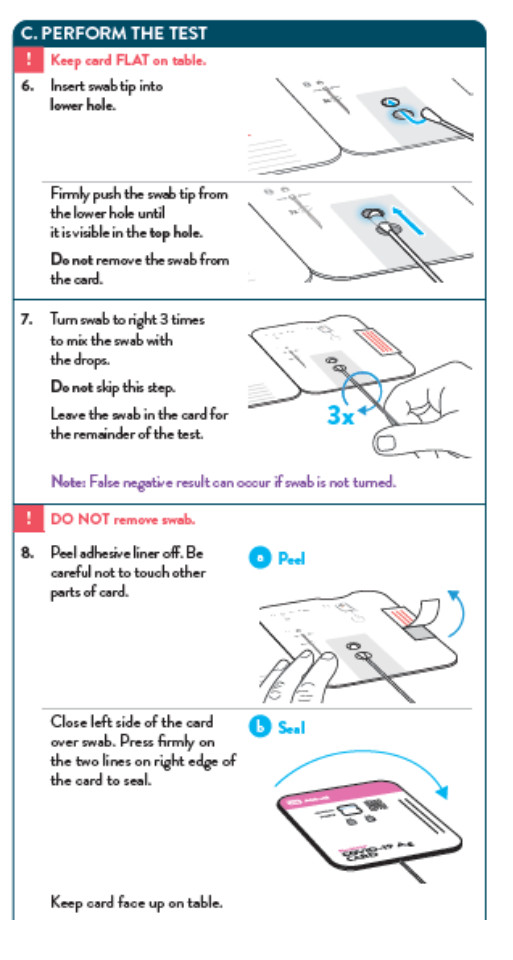
To perform the test, and anterior nasal swab specimen is collected by the patient, then 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab well. The patient sample is inserted into the test card through the bottom hole of the swab well and firmly pushed upwards until the swab tip is visible through the top hole. The swab is rotated 3 times clockwise and the card is closed, bringing the extracted sample into contact with the test strip. Test results are interpreted visually at 15 minutes based on the presence or absence of visually detectable pink/purple-colored lines. Results should not be read after 30 minutes.
BinaxNOW COVID-19 Antigen Self Test instructions for use are provided as a paper copy within the test kit, available digitally via website link (www.Bina now-self-test.Abbott) or digitally via the NOVICA app downloaded to a compatible smartphone. Compatible smartphone includes Apple iPhone running Operation System (iOS): latest major version and two prior major versions (iPhone running iOS v12 or later), and Android Phones: latest major version and two prior major versions (Android phone running Android OS v9 or later).
REAGENTS AND MATERIALS
Materials ProvidedTest Cards (2): A cardboard, book-shaped hinged test card containing the test stripExtraction Reagent (2): Bottle containing <1 mL of extraction reagentNasal Swabs (2): Sterile swab for use with BinaxNOW COVID-19 Antigen Self TestPatient Instructions for Use (1)Individual Fact Sheet (1)
PRECAUTIONS
- For in vitro diagnostic use.
- This product has not been FDA cleared or approved but has been authorized by FDA under a EUA.
- Wear a safety mask or other face-covering when collecting anterior nares swab specimen from a child or another individual.
- Use of gloves is recommended when conducting testing.
- Keep testing kit and kit components out of the reach of children and pets before and after use.
- . This product has been authorized only for the detection of proteins from SARS-CoV-2, n1ot for any other viruses or pathogens.
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for the detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
- Proper sample collection and handling are essential for correct results.
- Do not use a kit that has been opened and/or tampered with.
- Leave test card sealed in its foil pouch until just before use. Do not use if the pouch is damaged or open.
- Do not dip the swab into the liquid reagent or other liquid before inserting the swab into the nose.
- Do not touch the swab tip when handling the swab sample.
- Do not use the kit past its expiration date.
- Do not mix components from different kit lots.
- All kit components are single-use items. Do not use with multiple specimens. Do not reuse the used test card or swab.
- Dispose of kit components and patient samples in household trash.
- INVALID RESULTS can occur when an insufficient volume of extraction reagent is added to the test card. To ensure delivery of adequate volume, hold the bottle vertically, 1/2 inch above the swab well, and add drops slowly.
- The Reagent Solution contains a harmful chemical (see table below). If the solution contacts the skin or eye, flush with copious amounts of water. If irritation persists, seek medical advice: https://www.poison.org/contact-us-or-1-800-222-1222.
| Chemical Name/CAS | GHS Code for each Ingredient | Concentration |
| Sodium Azide/26628-22-8 | Acute Tox. 2 (Oral), H300Acute Tox. 1 (De rma l), H31 0 | 0.0125% |
STORAGE AND STABILITY
Store kit between 35.6-86°F (2-30°C). Ensure all test components are at room temperature before use. The BinaxNOW COVID-19 Antigen Self Test is stable until the expiration date marked on the outer packaging and containers.
DIRECTIONS FOR RUNNING THE BINAXNOW COVID-19 AG CARD SELF TEST


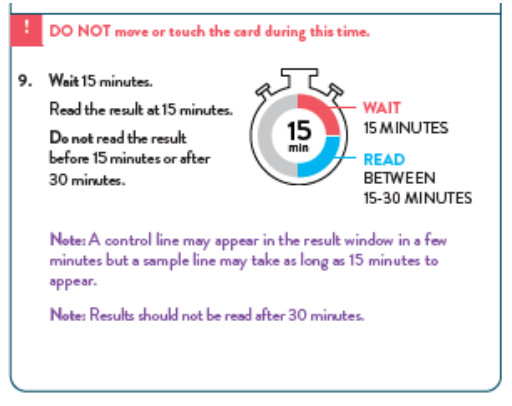

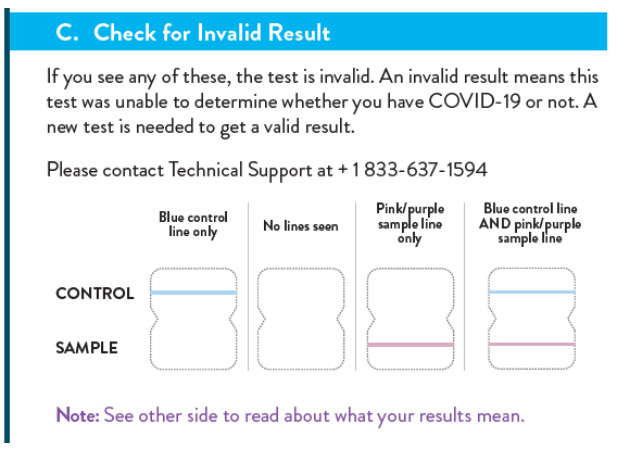
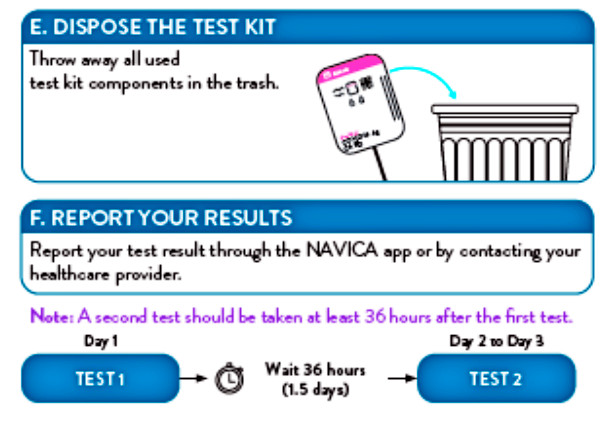
RESULT INTERPRETATION
Positive ResultA positive test result for COVID-19 indicates that antigens from SARS-CoV-2 were detected, and the patient is very likely to be infected with the virus and presumed to be contagious. Test results should always be considered in the context of clinical observations and epidemiological data (such as local prevalence rates and current outbreak/epicenter locations) in making a final diagnosis and patient management decisions. Patient management should follow current CDC guidelines.
Negative ResultA negative test result for this test means that antigens from SARS-CoV-2 were not present in the specimen above the limit of detection. However, a negative result does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. The amount of antigen in a sample may decrease as the duration of illness increases. Negative results should be treated as presumptive and confirmed with a molecular assay, if necessary, for patient management.
For serial testing programs, additional confirmatory testing with a molecular test for negative results may be necessary, if there is a high likelihood of COVID-19, such as an individual with close contact with COVID-19 or with suspected exposure to COVID-19 or in communities with a high prevalence of infection. Additional confirmatory testing with a molecular test for positive results may also be necessary if there is a low likelihood of COVID-19, such as in individuals without known exposures to COVID-19 or residing in communities with a low prevalence of infection.
LIMITATIONS
- This test detects both viable (live) and non-viable, SARS-CoV, and SARS-CoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- The performance of the BinaxNOW COVID-19 Antigen Self Test was evaluated using the procedures provided in this product insert only. Modifications to these procedures may alter the performance of the test.
- False-negative results may occur if a specimen is improperly collected or handled.
- False-negative results may occur if an inadequate extraction buffer is used (e.g., <6 drops).
- False-negative results may occur if specimen swabs are not twirled within the test card.
- False-negative results may occur if swabs are stored in their paper sheath after specimen collection.
- Positive test results do not rule out co-infections with other pathogens.
- False-negative results are more likely after eight days or more of symptoms.
- Positive test results do not differentiate between SARS-CoV and SARS-CoV-2.
- Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
- The presence of mupirocin may interfere with the BinaxNOW COVID-19 Antigen Self Test and may cause false-negative results.
- Negative results are presumptive, do not rule out COVID-19 infection and it may be necessary to obtain additional testing with a molecular assay if needed for patient management.
- Performance of nasal swabs collected from patients without symptoms or other epidemiological reasons to suspect COVID-19 infection or for serial screening, when tested twice over three days with at least 36 hours between tests has not been determined, a study to support use will be completed.
- If the differentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local public health departments, is required.
- The performance of this test was established based on the evaluation of a limited number of clinical specimens collected in November 2020. The clinical performance has not been established in all circulating variants but is anticipated to be reflective of the prevalent variants in circulation at the time and location of the clinical evaluation. Performance at the time of testing may vary depending on the variants circulating, including newly emerging strains of SARS-CoV-2 and their prevalence, which change over time.
PERFORMANCE CHARACTERISTICS
CLINICAL PERFORMANCE
Clinical performance characteristics of the BinaxNOW COVID-19 Antigen Self Test were evaluated in an ongoing multi-site prospective study in the U.S. A total of four (4) investigational sites throughout the U.S. participated in the study. To be enrolled in the study, patients had to be presenting at the participating study centers with suspected COVID-19 within 7 days of symptom onset. Each Subject was provided a BinaxNOW COVID-19 Antigen Self Test. Under the observation and coaching of a clinical site staff member trained as a proctor, the Subject self-collected one (1) nasal swab and performed the BinaxNOW COVID-19 Antigen Self Test. Test results were interpreted and recorded by the Subject or other home user and independently by the proctor. Parents of pediatric Subjects under the age of 14 or Legally Authorized Representatives of adult Subjects unable to perform self-collection collected one (1) nasal swab from the Subject, performed the BinaxNOW COVID-19 Antigen Self Test, then interpreted and recorded the result for the patient.
An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-CoV-2 was utilized as the comparator method for this study.
The performance of the BinaxNOW COVID-19 Antigen Self Test was established with 53 nasal swabs collected from individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW COVID-19 Antigen Self Test Performance within 7 days of symptom onset against the Comparator Method
|
BinaxNOW COVID-19Ag 2 Card Home Test |
Comparator Method |
||
| Positive Negative Total | Positive Negative Total | Positive Negative Total | |
|
Positive |
22 |
0 |
22 |
|
Negative |
2 |
28 |
30 |
|
Total |
24 |
28 |
25* |
| Positive Agreement: 22/24 91.7% (95% CI: 73.0% – 98.9%) | |||
| Negative Agreement: 28/28 100.0% (95% CI: 87.7% – 100.0%) |
*1 sample generated an invalid BinaxNOW COVID-19 Ag 2 Card result (0.1% invalid rate)
The performance of this test has not yet been clinically validated for use in patients without signs and symptoms of respiratory infection or for serial screening applications, and performance may differ in these populations.
Performance of BinaxNOW COVID-19 Antigen Self Test, with the test performed and results interpreted by the home user is similar to the performance obtained by test operators with no laboratory experience. Due to the relatively small sample size for the home use clinical study, at the time of the interim analysis, the BinaxNOW COVID-19 Antigen Self Test positive agreement established in this ongoing clinical study is estimated to be between 73.0% and 98.9% as reflected in the 95% Confidence Interval. This is consistent with the performance established in a separate multi-site study in the US, where the BinaxNOW COVID-19 Ag Card test was performed and results interpreted by test operators with no laboratory experience. In that study, BinaxNOW COVID-19 Ag Card test positive agreement was 84.6% (95% CI: 76.8% – 90.6%), refer below:
The performance of the BinaxNOW COVID-19 Ag Card was established with 460 nasal swabs collected from individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW COVID-19 Ag Card Performance within 7 days of symptom onset against the Comparator Method
|
BinaxNOW COVID-19Ag 2 Card Home |
Comparator Method |
||
|
Positive Negative Total |
Positive Negative Total |
Total |
|
|
Positive |
99 |
5 |
104 |
|
Negative |
18 |
338 |
356 |
|
Total |
117 |
343 |
460 |
| Positive Agreement: 99/117 84.6% (95% CI: 76.8% – 90.6%) | |||
| Negative Agreement: 338/343 98.5% (95% CI: 96.6% – 99.5%) |
Patient demographics, the time elapsed since the onset of symptoms for all patients enrolled in the above study, are presented in the table below. Positive results are broken down by days since symptom onset:
|
Days Since Symptom Onset |
Cumulative RT- PCR Positive (+) |
Cumulative BinaxNOW COVID-19Antigen Self Test Positive (+) |
PPA |
95 % Confidence Interval |
|
|
1 |
12 | 10 | 83.3% | 51.6% | 97.9% |
|
2 |
34 | 28 | 82.4% | 65.5% |
93.2% |
|
3 |
50 | 41 | 82.0% | 68.6% |
91.4% |
|
4 |
63 | 50 | 79.4% | 67.3% |
88.5% |
|
5 |
78 | 63 | 80.8% | 70.3% |
88.8% |
|
6 |
90 | 75 | 83.3% | 74.0% |
90.4% |
|
7 |
117 | 99 | 84.6% | 76.8% |
90.6% |
|
8 to io |
144 | 118 | 81.9% | 74.7% |
87.9% |
|
11 tom |
161 | 126 | 78.3% | 71.1% | 84.4% |
|
All specimens |
167 | 129 | 77.2% | 70.1% |
83.4% |
A cohort of patients who presented with symptom onset greater than seven days was enrolled in the clinical study (n = 161). The positive agreement in patients with symptoms greater than seven days was 60% (30/50) and negative agreement was 98% (109/111). Therefore, negative results in patients with symptom onset greater than seven days should be interpreted with caution, as the sensitivity of the assay decreases over time.
ANALYTICAL PERFORMANCE Limit of Detection (Analytical Sensitivity)
BinaxNOW COVID-19 Antigen Self Test limit of detection (LOD) was determined by evaluating different concentrations of heat-inactivated SARS-CoV-2 virus. Presumed negative natural nasal swab specimens were eluted in PBS. Swab eluates were combined and mixed thoroughly to create a clinical matrix pool to be used as the diluent. Inactivated SARS-CoV-2 virus was diluted in this natural nasal swab matrix pool to generate virus dilutions for testing.Contrived nasal swab samples were prepared by absorbing 20 microliters of each virus dilution onto the swab. The contrived swab samples were tested according to the test procedure.The LOD was determined as the lowest virus concentration that was detected ≥ 95% of the time (i.e., concentration at which at least 19 out of 20 replicates tested positive).The BinaxNOW COVID-19 Antigen Self Test LOD in natural nasal swab matrix was confirmed 140.6 TCID50/mL.
| Concentration TCID50/mL | Number Positive/Total | % Detected |
| 140.6 | 20/20 | 100% |
Cross-Reactivity (Analytical Specificity) and Microbial Interference
Cross-reactivity and potential interference of BinaxNOW COVID-19 Antigen Self Test was evaluated by testing 37 commensal and pathogenic microorganisms (8 bacteria, 14 viruses, 1 yeast, and pooled human nasal wash) that may be present in the nasal cavity. Each of the organisms, viruses, and yeast were tested in triplicate in the absence or presence of heat-inactivated SARS-CoV-2 virus (45 TCID50/swab). No cross-reactivity or interference was seen with the following microorganisms when tested at the concentration presented in the table below.
|
Potential Cross-Reactant |
TestConcentration |
|
| Virus | Adenovirus | 1.0 x 10 TCID50 /mL |
| Human metapneumovirus (hMPV) | 1.0 x 105TCID50 /mL |
|
| Rhinovirus | 1.0 x 10 5 PFU/mL | |
| Enterovirus/Coxsackievirus B4 | 1.0 x 105 TCID50/mL | |
| Human coronavirus OC43 | 1.0 x 10 5 TCID50 /mL | |
| Human coronavirus 229E | 1.0 x 10 5 TCID50/mL | |
| Human coronavirus NL63 | 1.0 x 10 5 TCID50/mL |
| Human parainfluenza virus 1 | 1. 0 x io5TCID,,/mL | |
| Human parainfluenza virus2 | 1.0 X io5TCID,/mL | |
| Human parainfluenza virus 3 | to x io5TCID,/mL | |
| Human parainfluenza virus 4 | to x 1o5TCIDclmL | |
| influenza A | to x io5TCID,„/mL | |
| Influenza B | to x 1o5TCIDclmL | |
| Respiratory Syncytial Virus A | 1.0 x 105 PFU/mL | |
| Bacteria | Bordetellapertussis | 1.o x 106 cells/mL |
| Chlamydia pneumoniae | 1.o x 106 IFU/mL | |
| Haemophilus influenza | 1.o x 106 cells/mL | |
| Legionellapnuemophila | 1.0 x 106 cells/mL | |
| Mycoplasmapneumoniae | is x 106 U/mL | |
| Streptococcus pneumoniae | 1.o x 106 cells/mL | |
| Streptococcus pyogenes (groupA) | 1.0 x 106 cells/mL | |
| Mycobacterium tuberculosis | 1.o x 106 cells/mL | |
| Staphylococcus aureus | 1.0 x 106 org/mL | |
| Staphylococcus epidermidis | to x 106 org/mL | |
| Pooled human nasal wash | N/A | |
| Yeast | Candida albicans | 1.0 x 106 cells/mL |
To estimate the likelihood of cross-reactivity with SARS-CoV-2 virus in the presence of organisms that were not available for wet testing, In silico analysis using the Basic Local Alignment Search Tool (BLAST) managed by the National Center for Biotechnology Information (NCBI) was used to assess the degree of protein sequence homology.
- For P. jirovecii one area of sequence similarity shows 45% homology across 18% of the sequence, making cross-reactivity in the BinaxNOW COVID-19 Antigen Self Test highly unlikely.
- No protein sequence homology was found between M. tuberculosis, and thus homology-based cross-reactivity can be ruled out
- The comparison between SARS-CoV-2 nucleocapsid protein, MERS-CoV, and human coronavirus HKU1 revealed that cross-reactivity cannot be ruled out. Homology for KHU1 and MERS-CoV is relatively low, at 37.8% across 95% of the sequence and 57.14% across 87% of the sequence, respectively.
High Dose Hook Effect
No high dose hook effect was observed when tested with up to a concentration of 1.6 x 10 5 TCID50/mL of heat-inactivated SARS- CoV-2 virus with the BinaxNOW COVID-19 Antigen Self Test.
Endogenous Interfering Substances
The following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal cavity or nasopharynx, were evaluated with the BinaxNOW COVID-19 Antigen Self Test at the concentrations listed below and were found not to affect test performance.
| Substance | Active Ingredient | Concentration |
| Endogenous | M u cin2% w/v | |
| Whole Blood | 1% v/v | |
| OTC Nasal Drops | Phenylephrine | 15% v/v |
| OTC Nasal Gel | Sodium Chloride (i.e. NeilMed) | 5% v/v |
| OTC Nasal Spray 1 | Cromolyn | 15% v/v |
| OTC Nasal Spray 2 | Oxymetazoline | 15% v/v |
| OTC Nasal Spray 3 | Flu conazole | 5% w/v |
| Throat Lozenge | Be nzocaine, Menthol | 0.15% w/v |
| OTC Homeopathic Nasal Spray 1 | Galphimiaglauca, Sabadilla, | 2o% v/v |
| OTC Homeopathic Nasal Spray 2 | Zincumgluconium (i.e., Zicam) | 5% w/v |
| OTC Homeopathic Nasal Spray 3 | Alkalol | io% v/v |
| OTC Homeopathic Nasal Spray 4 | Fluticasone Propionate | 5% v/v |
| Sore Throat Phenol Spray | Phenol | 15% v/v |
| Anti-viral Drug | Tamiflu (Oseltamivir Phosphate) | 0.5% w/v |
| Antibiotic, Nasal Ointment | Mupirocin | 0.25% w/v |
| Antibacterial, Systemic | Tobramycin | 0.0004% w/v |
Usability Study
Abbott conducted a study to evaluate whether a home user can follow instructions and successfully perform the test steps for the BinaxNOW COVID-19 Antigen Self Test, including nasal swab collection at home, and correctly interpreting the results.
100 home users, including individuals (n=50) and caregivers (n=50), participated in the study. Each individual or caregiver pair participated in a 60-minute session with a single proctor. The usability evaluation session included one simulated use of the BinaxNOW COVID-19 Antigen Self Test and opportunities to provide feedback.
92% (92 out of 100) of home users produced a valid result (all negative) and 8 participants produced an invalid result.
100% (99 out of 99) of the home (individual and caregiver) participants correctly understood that failure to follow the test steps correctly would potentially lead to an invalid or inaccurate result or would require another test or consultation with a healthcare provider. (One participant was inadvertently not asked this question by the moderator during the session).
instruction Usability Study
The sponsor also submitted a usability study for the eInstruction. The goal of the usability study was to demonstrate that lay users can use paper instructions or digital (mobile app or website) instructions (i.e., paper Quick Reference Guide (QRG), digital app Quick Reference Instructions (QRI), or website electronic Instructions for Use (EU)) to perform the test steps for the BinaxNOW COVID-19 Antigen Self Test successfully.The study was conducted at usability labs in Chicago, IL, the USA from June 15 – June 23, 2021. A total of 60 lay users, including individuals (n=30) and caregivers (n=30), participated in the study. Each individual or caregiver pair participated in a 6-minute session with a study moderator. The usability evaluation session included one simulated use of the BinaxNOW COVID-19 Antigen Self Test, knowledge tasks, and opportunities to provide feedback.
SYMBOLS
| This symbol indicates that the product is for single use only. It is not to be re-used. | |
| This symbol indicates that you should consult the instructions for use. | |
| This symbol indicates that the product has a temperature limitation. | |
| This symbol indicates the name and location of the product manufacturer. | |
| This symbol indicates the product’s catalog number. | |
| For In Vitro Diagnostic Use. | |
| This symbol indicates that the total number of tests provided in the kit box |
FAQS
Our BinaxNOW Self Tests can provide the confidence needed to continue engaging in your communities as other COVID-19 safety measures begin to dissipate. Testing before and after events, school, work or social engagements gives you and your loved ones confidence, especially if you have loved ones who are at a higher risk.
Using the BinaxNOW Self Test is simple, even if you have never tested yourself before. You simply will perform a lower nostril nasal swab (not the deeper nasopharyngeal swab) and everything you need (swab, test card and reagent solution) is included in the box. Each test kit comes with an illustrated quick reference guide to walk you through the process step by step.
The test is indicated for all people aged 15 years or older and for children as young as 2 years old when samples are collected by an adult. The test can be used for people with and without symptoms.
Testing will remain an essential part of our short- and long-term COVID-19 recovery strategy. The BinaxNOW Self Test will be a key tool alongside vaccination as we get back to life. We don’t yet know how long vaccines confer immunity and how variants will evolve.
People can now self-report test results through our NAVICA app. In addition, people are encouraged to follow the latest CDC guidelines, which is to communicate your results to your healthcare provider, who is responsible for reporting your test results to the state health department.
There are two tests (as well as two swabs and reagents) in each box.
The tests should be administered twice over three days with at least 24 hours (and no more than 48 hours) between tests.
You can recycle the box, but should dispose of the test card, nasal swab and test solution in common household waste, in line with the test’s instructions for use.
o check for a positive result, look at the result window for two pink or purple lines. Even a faint line next to the word “sample” on the test card is a positive result. A negative result will have only one pink or purple line on the top half of the results window where it says “control.”
To check for a positive result, look at the result window for two pink or purple lines. Even a faint line next to the word “sample” on the test card is a positive result. A negative result will have only one pink or purple line on the top half of the results window where it says “control.”
Video
Technical Support Advice LineUS= +1 833 637 1594[email protected]
![]() Abbott Diagnostics Scarborough, Inc.10 Southgate Road , Scarborough, Maine 04074 USAwww.abbott.com/poct
Abbott Diagnostics Scarborough, Inc.10 Southgate Road , Scarborough, Maine 04074 USAwww.abbott.com/poct
© 2021 Abbott. All rights reserved.All trademarks referenced are trademarks of either the Abbott group of companies or their respective owners.IN195150 Rev. 2 2021/08
References
[xyz-ips snippet=”download-snippet”]