SARS-CoV-2 Antigen Rapid Test Kit(Colloidal Gold)Instructions for Use
【PRODUCT NAME】
SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold)
【PACKAGE AND SPECIFICATION】20 Tests/box (1Test/bag ×20 Bags) ,40 Tests/box (1Test/bag ×40 Bags)
【INTENDED USE】
For in vitro qualitative detection of SARS-CoV-2 nucleocapsidantigen in nasal (NS) swab specimens directly from individuals who are suspected of COVID-19 by their healthcare provider within the first 5 days after onset of symptoms. This test is only provided for use by clinical laboratories or to healthcare workers for point-of-care testing, not for at-home testing. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, or 2019-nCoV) is an enveloped non-segmented positive-sense RNA virus. It is the cause of coronavirus disease (COVID-19), which is contagious inhumans. SARS-CoV-2 has several structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N). The antigen is generally detectable in upper respiratory samples during the acute phase of infection. Positive results indicate the presence of viral antigens, but the clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out a bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results should be treated as presumptive, which do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
For in vitro diagnostic use only. For professional use only.
【TEST PRINCIPLE】
JOYSBIO Biotechnology’s SARS-CoV-2 Antigen Rapid Test Kit uses an immunocapture method, it is designed to detect the presence or absence of SARS-CoV-2 nucleocapsid proteins in respiratory samples from patients with signs and symptoms of infection who are suspected of COVID-19. Key components: the anti-nucleocapsid protein antibody and chicken IgY labeled by colloidal gold, the nitrocellulose membrane coated with anti nucleocapsid protein antibody, andgoat anti-chicken IgY antibody. When specimens are processed and added to the test device, SARS CoV-2 antigens present in the specimen bind to antibodies conjugated to colloidal gold in the test strip. The antigen-conjugate complexes migrate across the test strip to the reaction area and are captured by a line of antibodies bound on the membrane. A color band will show up when antigen-conjugate is deposited at the Test “T” position and the Control “C” position on the device.
【COMPONENT】
Materials provided:
Materials required but not provided with the kit:
【STORAGE AND STABILITY】
- Store at 2~30ºC in the sealed pouch up to the expiration dateand the validity is tentatively 24 months. Do not freeze.
- The test cassette should be used within 1 hour after taking out from the aluminum foil bag.
- Keep away from sunlight, moisture, and heat.
【SPECIMEN COLLECTION AND HANDLING】
- Specimen Collection and Preparation Acceptable specimens for testing with this kit include nasal swab specimens obtained by the dual nares collection method. Correct specimen collection and preparation methods must be followed. Specimens obtained early during symptom onset will contain the highest viral titers; specimens obtained after five days of symptoms are more likely to produce negative results when compared to an RT-PCR assay. Inadequate specimen collection, improper specimen handling and/or transport may yield a falsely negative result; therefore, training in specimen collection is highly recommended due to the importance of specimen quality for generating accurate test results.
- Specimen Transport and Storage Freshly collected specimens should be processed as soon as possible, but no later than one hour after specimen collection. Correct specimen collection and preparation methods must be followed.
- Nasal Swab Specimen Collection
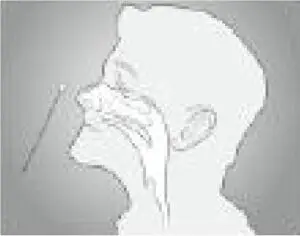
a. Insert the swab into one nostril of the patient. The swab tip should be inserted up to 2.5 cm (1 inch) from the edge of the nostril. Roll the swab 5 times along the mucosa inside the nostril to ensure that both mucus and cells are collected.
b. Using the same swab, repeat this process for the other nostril to ensure that an adequate sample is collected from both nasal cavities.
 c. Withdraw the swab from the nasal cavity. The sample is now ready for processing using the kit.
c. Withdraw the swab from the nasal cavity. The sample is now ready for processing using the kit. - DOs and DON’Ts of Sample Collection
- Do collect samples as soon as possible after the onset of symptoms.
- Do test samples immediately.
- Use only swabs provided with the kit.
- Do not place the swab back into the swab packaging sleeve after specimen collection.
【TEST PROCEDURE】
- The test kit, the specimen must be at room temperature (15~30ºC) for before testing. The kit is only intended for nasal swab specimens that are collected and tested directly (i.e., swabs that have NOT been placed in transport media). The kit includes a pre-diluted processing reagent in a ready to use buffer bottle. This kit IS NOT INTENDED for testing liquid samples such as a wash or aspirate samples or swabs in transport media as results can be compromised by over dilution.
- Freshly collected specimens should be processed within 1hour.
•Step 1: Twist of the top of the buf er bottle, slowly dispense all of the buf er into the extraction Tube.
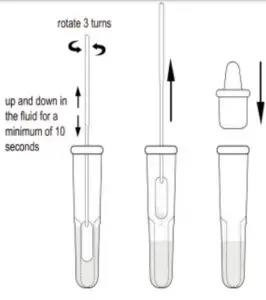
•Step 2: After collection of nasal (NS) swab specimen, insert the swab into the tube and plunge theswab up and down in the fluid for a minimum of 20 seconds, then hold the swab against the bottom of the tube and roll 5 turns, taking care not to splash contents out of the tube.
•Step 3: Remove the swab while squeezing the sides of the tube to tract the liquid from the swab.
•Step 4: Press the nozzle cap firmly onto the extraction tube containing the processed sample (threading or twisting is not required). Mix thoroughly by swirling or flicking the bottom of the tube. Place the extraction tube(s) in a rack in the designated area of the workspace.

•Step 5Tear of the foil pouch, take out the test strip/cassette and place the test kit on a cleanand level surface. Label the test device and one extraction tube for each specimen or control to be tested.
•Step 6Gently squeeze the ridged body of the tube, dispensing three (3) drops of the processed specimen into the sample well.
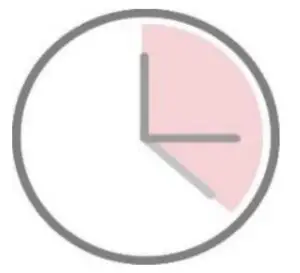
•Step 7Read the test results between 15 and 20 minutes. Do not read the results after 20 minutes.
NOTE: Do not use tubes or tips from any other product, orfrom other manufacturers.
【INTERPRETATION OF TEST RESULTS】
1.POSITIVE:
Two lines appear. A colored line should be in the control line region (C), a colored line appears in the test line (T) region. Positive results indicate the presence of viral antigens, but the clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out a bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
2.NEGATIVE:
Only one colored control line appears. Negative results are presumptive. Negative test results do not preclude infection and should not be used as the sole basis for treatment or other patient management decisions, including infection control decisions, particularly in the presence of clinical signs and symptoms consistent with COVID-19, or in those who have been in contact with the virus. It is recommended that these results be confirmed by a molecular testing method, if necessary, for patient management.
3.INVALID:
Control line fails to appear. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
4.Result determination time:
The result should be judged within 15~20 minutes after the sample is added into the sample well, and the result displayed after 20 minutes is invalid.

(The picture is for reference only)
【LIMITATIONS OF TEST METHOD】
- This product is only suitable for a qualitative test and auxiliary diagnosis.
- The test results are only for clinical reference and should not be the only basis for clinical diagnosis and treatment. The clinical management of patients should be considered in combination with their symptoms, physical signs, medical history, other laboratory tests, therapeutic reaction, and epidemiological information.
- Users should test specimens as quickly as possible after specimen collection.
- Positive test results do not rule out co-infections with other pathogens.
- Results from the test should be correlated with the clinical history, epidemiological data, and other data available to the clinician evaluating the patient.
- A false-negative test result may occur if the level of viral antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly; therefore, a negative test result does not eliminate the possibility of SARS-CoV-2 infection.
- The amount of antigen in a sample may decrease as the duration of illness increases. Specimens collected after day 5 of illness are more likely to be negative compared to an RT-PCR assay.
- Failure to follow the test procedure may adversely affect test performance and/or invalidate the test result.
- The contents of this kit are to be used for the qualitative detection of SARS CoV-2 antigens from nasal swab specimens only.
- The kit performance depends on antigen load and may not correlate with other diagnostic methods performed on the same specimen.
- Negative test results are not intended to rule in other non SARS-CoV-2 viral or bacterial infections.
- Positive and negative predictive values are highly dependent on prevalence rates. Positive test results are more likely to represent false-positive results during periods oflittle/no SARS-CoV-2 activity when disease prevalence is low. False-negative test results are more likely when the prevalence of disease caused by SARS-CoV-2 is high.
- This kit has been evaluated for use with human specimen material only.
- Monoclonal antibodies may fail to detect or detect with less sensitivity, SARS-CoV-2 viruses that have undergone minor amino acid changes in the target epitope region.
- The performance of this test has not been evaluated for usein patients without signs and symptoms of respiratory infection and performance may differ in asymptomatic individuals.
- The sensitivity of the test after the first five days of the onset of symptoms has been demonstrated to decrease as compared to an RT-PCR SARS-CoV-2 assay.
- Negative results should be treated as presumptive and confirmed with an FDA authorized molecular assay, if necessary, for clinical management, including infectioncontrol.
- Specimen stability recommendations are based upon stability data from influenza testing and performance may be different from SARS-CoV-2. Users should test specimens as quickly as possible after specimen collection, and within one hour after specimen collection.
- The validity of the kit has not been proven for identification/confirmation of tissue culture isolates and should not be used in this capacity
【PERFORMANCE CHARACTERISTICS】
- Clinical PerformanceThe performance of the kit was established with 150 direct nasal swabs prospectively collected and enrolled from individual symptomatic patients who were suspected of COVID-19. As with all antigen tests, performance may decrease as days since symptom onset increases. Samples were collected by qualified personnel in China. Nasal swabs were collected following the dual nares method and handled as described in the instruction of the kit. Specimens were frozen within 30 minutes of collection and stored until tested. All specimens within a pre-specified date range were selected and then sequentially tested in a blinded fashion. The performance of the kit was compared to results of a nasopharyngeal or oropharyngeal swab tested with a commercialized molecular assay. The kit showed 88.89% of sensitivity and 99.05% of specificity. Table 1. Clinical Study Results from symptom onset
 Positive Percent Agreement (PPA)= 40/45(88.89%) (95%CI: 75.9%~96.3%)Negative Percent Agreement (NPA)= 104/105(99.05%)(95%CI:94.8%~100.0%)Accuracy=(40+104)/150×100%=96.00%Kappa=2×4155/ 9210=0.90>0.5
Positive Percent Agreement (PPA)= 40/45(88.89%) (95%CI: 75.9%~96.3%)Negative Percent Agreement (NPA)= 104/105(99.05%)(95%CI:94.8%~100.0%)Accuracy=(40+104)/150×100%=96.00%Kappa=2×4155/ 9210=0.90>0.5 - Assay Cross-ReactivityCross-Reactivity: There was no cross-reaction with potential cross-reactive substances except SARS-coronavirus.Table 2: Cross-reactivity Results



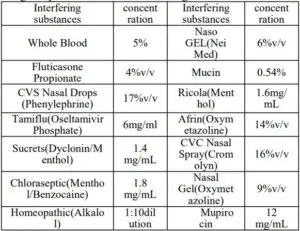
- .Potentially Endogenous Interfering SubstancesSARS-CoV-2 Antigen nasal swab samples were spiked with one of the following substances to specified concentrations and tested in multiple replicates. No false positivity or false negativity was found with the following:


- Limit of Detection(ANALYTICAL SENSITIVITY)The LOD for the SARS-CoV-2 antigen rapid test kit is 1.6 x10 2TCID50/ mL. The LOD for the SARS-CoV-2 antigen rapid test kit was established using limiting dilutions of a viral sample inactivated by gamma irradiation. The material was supplied at a concentration of 1.3 x 10 6 TCID50/mL. In this study, designed to estimate the LOD of the assay when using a direct nasal swab, the starting material was spiked into a volume of virus dilution in saline. An initial range-finding study was performed testing devices in triplicate using a 10-fold dilution series. At each dilution, 50 μL samples were added to swabs and then tested using the procedure appropriate for patient nasal swab specimens. A concentration was chosen between the last dilution to give 3 positive results and the first to give 3 negative results. Using this concentration, the LOD was further refined with a 2-fold dilution series. The last dilution demonstrating 100% positivity was then tested in an additional 20 replicates tested in the same way.
- Hook Effect:As part of the LoD study, the highest concentration of thesample (1.3 x 10 6 TCID50/mL) was tested. There was no Hook effect detected.
【WARNINGS】
- A negative result can occur if the SARS-CoV-2 virus presentin the specimen is below the sensitivity of the kit.
- Not for the screening of donated blood.
- Do not smoke, drink, or eat in areas where specimens or kit reagents are being handled.
- Dispose of all specimens and materials used to perform thetest as biohazardous waste.
- Handle the negative and positive controls in the same manner as patient specimens for operator protection.
- Do not perform the test in a room with strong airflow, i.e. anelectric fan or strong air-conditioning.
【EXPLANATION OF LABELS】
【BASIC INFORMATION】
![]() JOYSBIO(Tianjin) Biotechnology Co., Ltd. Address: Tianjin International Joint Academy of Biotechnology& Medicine 9th floor No.220, Dongting Road,TEDA 300457 Tianjin China Tel:+86-022-65378415
JOYSBIO(Tianjin) Biotechnology Co., Ltd. Address: Tianjin International Joint Academy of Biotechnology& Medicine 9th floor No.220, Dongting Road,TEDA 300457 Tianjin China Tel:+86-022-65378415
![]() Lotus NL B.V. Address: Koningin Julianaplein 10,le Verd,2595AA, The Hague,Netherlands.
Lotus NL B.V. Address: Koningin Julianaplein 10,le Verd,2595AA, The Hague,Netherlands.
【DATE OF APPROVALAND AMENDMENT OF IFU】: March-2020
SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold) Instructions for Use – SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold) Instructions for Use –
[xyz-ips snippet=”download-snippet”]


