DeVilbiss 7310 Compact Suction Unit

CAUTION–Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. Made in U.S.A. of US & Imported Parts.
IMPORTANT SAFEGUARDS
When using electrical products, especially when children are present, basic safety precautions should always be followed. Read all instructions before using. Important information is highlighted by these terms:DANGER–Urgent safety information for hazards that will cause serious injury or death.WARNING–Important safety information for hazards that might cause serious injury.CAUTION–Information for preventing damage to the product.NOTE–Information to which you should pay special attention.
READ ALL INSTRUCTIONS BEFORE USING. Save These Instructions
DANGER
To reduce the risk of electrocution:
- Do not use while bathing.
- Do not place or store product where it can fall or be pulled into a tub or sink.
- Do not place in or drop into water or other liquid.
- Do not reach for a product that has fallen into water. Unplug immediately.
Warning
To reduce the risk of burns, electrocution, fire or injury to persons:
- Close supervision is necessary when this product is used by, on, or near children or physically incapacitated individuals.
- Use this product only for its intended use as described in this guide.
- Never operate this product if:
- It has a damaged power cord or plug.
- It is not working properly.
- It has been dropped or damaged.
- It has been dropped into water. Return the product to an authorized DeVilbiss service center for examination and repair.
- Keep the power cord away from heated surfaces.
- Never use while drowsy or asleep.
International TravelThe suction unit is equipped with an AC to DC Adapter/Charger allowing operation on any AC voltage (100-240 VAC, 47-63 Hz). However the correct power cord must be used to connect to adaptable wall power. Your unit is approved for use on commercial aircraft; specific standards are listed in the Specifications in this guide. Certificate is available upon request; if needed, contact your provider for a copy of approval letter before travel.NOTE-Check power cord for adaptability before using.
IntroductionYour DeVilbiss Suction Unit is a compact medical suctioning device which has been designed for reliable, portable operation. Following the recommended operating and maintenance procedures outlined in this instruction guide will maximize the life of this product.
Intended Use StatementThe device is to be used to remove fluids from the airway or respiratory support system and infectious materials from wounds. The device creates a negative pressure (vacuum) that draws fluids through disposable tubing that is connected to a collection container. The fluids are trapped in the collection container for proper disposal. It is for use on the order of a physician only.
ContraindicationsThe Vacu-Aide Compact Suction Unit should not be used for:
- thoracic drainage
- nasogastric suction
DANGER
The DeVilbiss Vacu-Aide is a vacuum suction device designed for the collection of nonflammable fluid materials in medical applications only. Improper use during medical applications can cause injury or death. For all medical applications:
- All suctioning should be done in strict accordance with appropriate procedures that have been established by a licensed medical authority.
- Some attachments or accessories may not fit the tubing supplied. All attachments or accessories should be checked prior to use to assure proper fit.
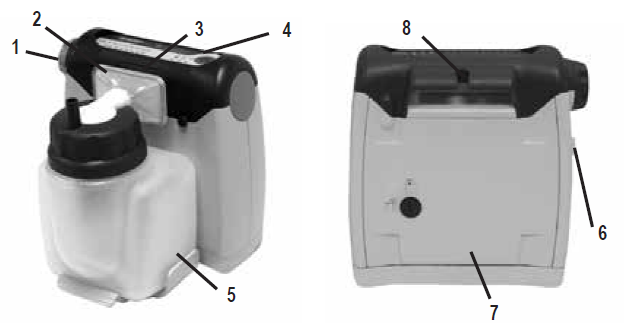
IMPORTANT PARTS
Inspect the suction unit and all parts before use.
- Vacuum Regulator Knob (on side)
- Bacteria Filter (non-sterile)
- Vacuum Inlet Port (behind filter)
- Display Panel (top of unit)
- 725 ml Reusable Container with Lid/ElbowAssembly
- DC Power Input (on side)
- Battery Door
- Unit Carry Handle/Catheter Holder
- 6’ (1.8m) Patient Tubing (not shown)
- AC to DC Adapter/Charger (not shown)
- 12V DC Power Cord (not shown)
- High Capacity Rechargeable Battery (not shown)
- Carry Case with Shoulder Strap (not shown)

How To Use:
Accessory/Replacement Items
The following items can be purchased separately as accessories or replacement for your suction unit.
| Description | Part No. | Description | Part No. |
| Bacteria Filter (12 pack) | 7305D-608 | 12V DC Power Cord | 7304D-619 |
| Battery Door | 7310P-602 | Line Cord – Hospital Grade USA | 099HD-614 |
| AC to DC Adapter/Charger | 7305P-613 | Line Cord USA | 6710D-609 |
| 6’ Patient Tubing | 6305D-611 | Line Cord UK | 7305P-630 |
| Carrying Case w/Shoulder Strap | 7310P-606 | Line Cord EU | 7305P-631 |
| 725 ml Reusable Container Pkg (Jar, Lid/ Elbow Assembly, Filter) | 7310P-603 |
Set-Up
- Your unit was shipped with the battery disconnected. Connect battery and charge before first use. Refer to Battery Replacement section for instructions.
- Place clear side of filter labeled IN directly into container’s lid.
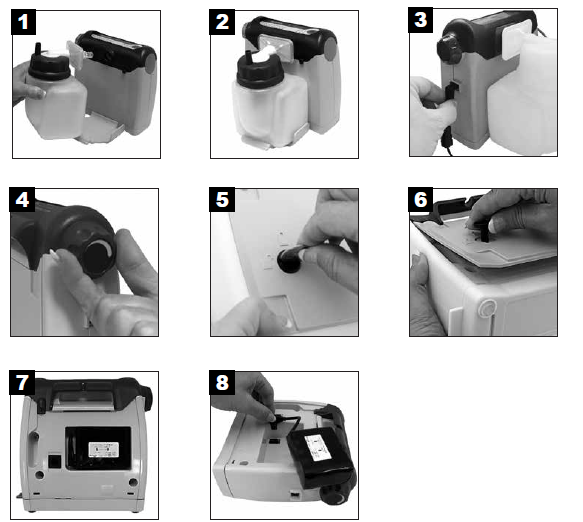
- With filter attached to container, directly insert into the front port. Both the filter and the bottom support secure the container to the unit (FIG. 1 & 2).
- The 6’ (1.8m) patient tubing can now be connected to the container lid at the outlet labeled <Patient>.
- Ensure that all connections are secure before use to prevent leaks.
- Verify that the unit is at the desired suction level before beginning patient suctioning.
How to Operate Your Suction Unit
Control Panel Symbols
Power Source Options
AC OPERATION – Plug the 90° power connector of AC to DC adapter/charger into DC power input (FIG 3) and attach the line cord. Plug the other end of AC adapter into a grounded AC supply.12V DC OPERATION – Plug the small 90° power connector of DC power cord into DC power input (FIG 3). Plug large end of cord into 12V DC power receptacle of vehicle.BATTERY OPERATION – Your unit is equipped with a high capacity rechargeable battery. For initial charge on new unit, fully charge the battery for a minimum of 5 hours (see Battery Charging). To operate unit from the rechargeable battery, ensure that no external power sources are plugged into the suction unit.NOTE– During charging or operating, the power supply may become warm to touch; this is normal.
warningIf the unit does not receive external power or the battery has not been charged, the low battery indicator light will remain on and the performance of the unit will drop rapidly. If Low Battery symbol lights, switch to another power source to avoid an interrupted suction procedure.
How to adjust the vacuum level
- Once power source is selected, turn the unit on by pressing the “On” button. The GREEN light, indicating external power, will remain lit when external power is connected.
- Occlude (block) the patient end of the tubing, then adjust vacuum level from 50-500 mmHG +/- 10% by turning the vacuum regulator knob clockwise to increase and counter-clockwise to decrease the vacuum (FIG 4). Release and occlude once more to confirm setting. The desired level of vacuum can be viewed on the LED display.NOTE–The LEDs have two brightness levels. As the vacuum level is adjusted, the LEDs will illuminate in progression. When an LED is at half brightness, it indicates that the vacuum level is halfway between the previous fully lit LED and the half brightness LED. EXAMPLE: If the 150 mmHg LED is fully illuminated and the 200 mmHg LED is at half brightness, the suction level is 175 mmHg. When the 200 mmHg LED illuminates at full brightness, the unit has reached 200 mmHg.
- Connect suction tip or catheter as appropriate.NOTE– If the unit does not maintain vacuum, refer to Troubleshooting.NOTE– Suction ceases if liquid level reaches float shut-off valve located on underside of container lid.CAUTION–Further suctioning may cause damage to the vacuum pump and voids warranty. Equipment service is required if fluid content is aspirated back into the unit.
Battery Charging
The Vacu-Aide Compact series is equipped with a factory-installed high capacity rechargeable battery. Connect battery and charge before first use (refer to Battery Replacement).
- Connect the unit to either an AC or DC power source.
- The green external power light should illuminate; the yellow charge indicator will remain lit while the battery is charging.
- Ensure that the yellow charging light is illuminated when charging begins. As the battery nears a full charge, the yellow battery charging light may flash for several minutes. This is normal. If your unit does not hold a charge, check that the yellow light turns on when external power is applied with the power button “Off.” If problems persist, contact your medical equipment provider.
NOTE–A fully discharged battery will require up to 5 hours (depending on depth of discharge) of charging to reach full capacity. If unit is not in use for extended periods, the battery should be recharged a minimum of every 6 months. A fully charged battery will provide approximately 45-60 minutes of continuous operation at zero vacuum level (free flow).CAUTION–Completely discharging the battery will shorten the battery life. Do not operate the unit for more than a few minutes if the low battery indicator light is lit. Recharge the battery as soon as possible
Battery replacement
- Using a coin or straight-blade screwdriver rotate latch to unlocked position (FIG. 5).
- Remove door by pulling up on latch (FIG. 6).
- Remove battery from compartment and unplug connector from circuit board (FIG. 7 & 8).
- Install new battery by reversing the above steps.
- Fully charge battery before using
Cleaning Instructions
Collection Container:
NOTE–Elbow is permanently attached to the lid and cannot be removed.
- Push power button to turn unit off. Wait for vacuum level to drop.
- Disconnect external power source from input receptacle on unit (if applicable).
- Remove container from unit by disconnecting the patient tubing and filter.NOTE–The reusable collection container and lid are single-patient use and should be replaced every 1 to 2 months, depending on use and cleaning method.
- Collection containers and lids should be emptied and thoroughly cleaned after each use. Wash in a warm water/ dishwashing detergent solution and rinse with clean, warm tap water. Then soak with a commercial disinfectant or one part vinegar (>=5% acetic acid concentration) to three parts warm water (131ºF-149ºF/55ºC-65ºC) solution for 60 minutes. Thoroughly rinse with warm tap water and air dry. Note: If using a commercial disinfectant, follow manufacturer’s recommended instructions and dilution ratios carefully.
Suction Unit (single-patient use)
- With the power “Off,” disconnect the unit from all external power sources.
- Wipe the outside housing with a clean cloth dampened with commercial disinfectant/detergent (bacterial-germicidal).CAUTION– Do not submerge suction unit in water; this will result in damage to vacuum pump.
Tubing (single-patient use)
- Disconnect the tubing from the unit.
- Rinse thoroughly by running warm tap water through it.
- Follow by soaking in a solution of 1 part vinegar (>=5% acetic acid concentration) to 3 parts water (131ºF-149ºF/55ºC-65ºC) for 60 minutes. Rinse with clean, warm water and air dry.
- Keep the outer surface of the tubing clean by wiping with a clean, damp cloth.
Carrying Case (single-patient use)Wipe the case using a clean cloth dampened with detergent and/or disinfectant.Note– Disinfection information is based on AARC Clinical Practice Guideline Suctioning of the Patient in the Home.
Suction Unit (multi-patient use)Device Cleaning and Disinfection When There is a Patient Change When medical devices have already been used with a patient, contamination with human pathogenic germs should be assumed (unless there is evidence to the contrary), and the next patient, user or third party should be protected by appropriate handling and preparation. Therefore, when there is a patient change, people must be protected during the transport and handling of the device and the device must be fully processed, i.e., cleaned and disinfected, by suitably trained personnel before reuse to protect the next patient. The complete processing may only be done by the manufacturer or by a qualified DeVilbiss provider/service technician.Note – When the unit is used as per instructions under normal conditions the interior of the unit is protected from exposure to pathogens by the in-line filter on the collection container, hence no disinfection of internal components is necessary.Note – If the unit is used without an in-line filter then the interior of the unit has been exposed to pathogens and the unit cannot be disinfected.Note – If the following processing of the unit by a qualified DeVilbiss provider/technician is not possible, the unit must not be used by another patient!
DeVilbiss Healthcare recommends that at least the following procedures be carried out by the manufacturer or a qualified third party between uses by different patients.
- Dispose of all accessory components that are not suitable for reuse, i.e., collection container, filter, tubing and carrying case.
- With the power switch in the “Off” position, disconnect the DeVilbiss Suction Unit from all external power sources.
- Visually inspect unit for any damage, missing parts, etc.
- Wipe the housing with a clean cloth and a commercial (bacterial-germicidal) disinfectant that meets the requirements listed in the NOTE below and is used as per the disinfectant manufacturer’s recommended dilution rates and instructions.CAUTION – Do not submerge in water as this will result in damage to the vacuum pump.NOTE – Do not use any cleaners or disinfectants that contain ammonia, benzene and/or acetone to clean the unit.
Maintenance
Changing Filter & Replacing Container
- Change the bacteria filter every 1 to 2 months OR immediately if overflow occurs.
- Remove the bacteria filter by disconnecting it from the suction unit and lid assembly.
- Replace it with a new DeVilbiss bacteria filter # 7305D-608 (12 pack).NOTE–Verify clear side of filter marked <In> faces container.NOTE–Use only the bacteria filter provided by DeVilbiss Healthcare or one of its Distributors. Substitution may lead to contamination of the unit and/or poor performance and will void warranty.
- Replace Collection Container every 1 to 2 months, depending on use and cleaning method.NOTE– Use only DeVilbiss 725 ml reusable containers. Substitution may lead to poor performance and will void warranty.
Troubleshooting
NOTE– Before you contact your equipment provider, follow Troubleshooting below:
DangerElectric shock hazard. Do not attempt to open or remove cabinet, there are no user-serviceable internal components. If service is required, return the suction unit to a qualified DeVilbiss provider or authorized service center. Opening or tampering with the unit will void the warranty.NOTE– If problem is not resolved, contact your authorized equipment provider.
| Problem | Action |
| Unit does not power on. (Green external power indicator should be illuminated when power is applied.) | 1.Check power sources and connections.
2.Ensure wall outlet is live by plugging in a lamp. 3.Check that battery is fully charged. |
| Pump runs, but no vacuum. | 1.Check that all tubing is connected properly.
2.Check tubing connections for breaks, leaks, or occlusions. 3.Ensure that float shut-off is not activated due to full container. 4.Check for leaks or cracks in container assembly. |
| Low vacuum. | 1.Use vacuum adjustment knob to increase vacuum level.
2.Check system for leaks. |
| Battery will not hold a charge. | 1.Confirm battery is connected (refer to Set-Up).
2.Verify that charge light turns on. 3.Check electrical connections during charging. 4.Ensure wall outlet is live by plugging in a lamp. If problem continues, contact your equipment provider. |
Specifications/ClassificationsSize . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.25”H x 7.25”W x 6.75”D (18.4cm x 18.4cm x 17.1cm)Weight . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.375 lbs. (1.53 kg)Electrical Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .100-240 VAC 50/60 Hz 0.75 A max; 12 VDC, 33 W maxVacuum Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .50-500 mmHG +/- 10%Air Flow @ pump inlet: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27 LPM (free flow) typical(may be less when running from internal battery)Collection Container Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 725 ml (cc)
Environmental ConditionsOperating Temperature Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32°F (0°C) – 104°F (40°C)Operating Relative Humidity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .0-95%Operating Atmospheric Pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10.2 Psi (70 kPA) – 15.4 Psi (106 kPA)Storage & Transport Temperature Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . -40°F (-40°C) – 158°F (70°C)Storage & Transport Relative Humidity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .0-95%Storage & Transport Atmospheric Pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.3 Psi (50 kPA) – 15.4 Psi (106 kPA)
Warranty7310 Series . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Two-years limited, excluding internal battery and collection containerInternal Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 months
ApprovalsIEC 601-1; IEC 68; CAN/CSA-C22.2 No. 601.1-M90; UL 2601-1, CE EN 60601-1-2, ISO10079-1:1999Meets RTCA/DO-160E . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . DO-160E – section 21 Category M (for battery operation only; commercial aircraft, airborne equipment)
Equipment ClassificationsWith respect to protection from electric shock . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Class I and internally poweredDegree of protection against electric shock . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Type BF Applied PartsDegree of protection against ingress of liquids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .IP12 and ordinary power supplyMode of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Intermittent Operation: 30 minutes on, 30 minutes off Equipment not suitable for use in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide.
ISO Classification7310 Series – Electrically powered medical suction equipment for field and transport use according to ISO 10079-1 : 1999 High Flow/High Vacuum
Two-Year Limited Warranty
The compressor portion of the DeVilbiss Vacu-Aide Compact Suction Unit 7310 Series (excluding internal rechargeable battery and collection container) is warranted to be free from defective workmanship and materials for a period of two years from date of purchase. Internal rechargeable batteries are warranted for 6 months. Any defective part(s) will be repaired or replaced at DeVilbiss Healthcare’s option if the unit has not been tampered with or used improperly during that period. Make certain that any malfunction is not due to inadequate cleaning or failure to follow the instructions. If repair is necessary, contact your DeVilbiss Provider or DeVilbiss for instructions.NOTE – Be sure to retain a dated proof of purchase document to verify unit is within 2-year warranty period.NOTE – This warranty does not cover providing a loaner unit, compensating for costs incurred in rental while said unit is under repair, or costs for labor incurred in repairing or replacing defective part(s).
THERE IS NO OTHER EXPRESS WARRANTY. IMPLIED WARRANTIES, INCLUDING THOSE OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE, ARE LIMITED TO THE DURATION OF THE EXPRESS LIMITED WARRANTY AND TO THE EXTENT PERMITTED BY LAW. ANY AND ALL IMPLIED WARRANTIES ARE EXCLUDED. THIS IS THE EXCLUSIVE REMEDY AND LIABILITY FOR CONSEQUENTIAL AND INCIDENTAL DAMAGES UNDER ANY AND ALL WARRANTIES ARE EXCLUDED TO THE EXTENT EXCLUSION IS PERMITTED BY LAW. SOME STATES DO NOT ALLOW LIMITATIONS ON HOW LONG AN IMPLIED WARRANTY LASTS, OR THE LIMITATION OR EXCLUSION OF CONSEQUENTIAL OR INCIDENTAL DAMAGES, SO THE ABOVE LIMITATION OR EXCLUSION MAY NOT APPLY TO YOU. This warranty gives you specific legal rights, and you may also have other rights which vary from state to state.
Manufacturer’s NoteThank you for choosing a DeVilbiss Vacu-Aide Compact Suction Unit. We want you to be a satisfied customer. If you have any questions or comments, please send them to our address on the back cover. For Service Call Your Authorized DeVilbiss Provider:
Phone_ _______Purchase Date____Serial #_ ________
DeVilbiss guidance and manufacturer ’s Declaration
WARNINGMedical Electrical Equipment needs special precautions regarding EMC and needs to be installed and put into service according to the Electromagnetic Compatibility [EMC] information provided in the accompanying documents.Portable and Mobile RF Communications Equipment can affect Medical Electrical Equipment. The equipment or system should not be used adjacent to or stacked with other equipment and that if adjacent or stacked use is necessary, the equipment or system should be observed to verify normal operation in the configuration in which it will be used.NOTE– The EMC tables and other guidelines provide information to the customer or user that is essential in determining the suitability of the Equipment or System for the Electromagnetic Environment of use, and in managing the Electromagnetic Environment of use to permit the Equipment or System to perform its intended use without disturbing other Equipment and Systems or non-medical electrical equipment.
| Guidance and Manufacturer’s Declaration – Emissions All Equipment and Systems | ||
| This device is intended for use in the electromagnetic environment specified below. The customer or user of this device should assure that it is used in such an environment. | ||
| Emissions Test | Compliance | Electromagnetic Enforcement – Guidance |
| RF Emissions CISPR 11 |
Group 1 |
This device uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. |
| RF Emissions CISPR 11 | Class B
Radiated and Conducted Emissions |
This device is suitable for use in all establishments including domestic, and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. |
| Harmonics IEC 61000-3-2 | Class A | |
| Flicker
IEC 61000-3-3 |
Complies |
| Immunity Test | IEC 60601 Test Level | Compliance Level | Electromagnetic Environment – Guidance |
| Electrostatic Discharge (ESD) IEC 61000-4-2 | ±6kV contact
±8kV air |
±6kV contact
±8kV air |
Floors should be wood, concrete or ceramic tile. If floors are synthetic, the relative humidity should be at least 30%. |
| Electrical Fast Transient/burst IEC 61000-4-4 |
±2kV on AC Mains |
±2kV on AC Mains |
Mains power quality should be that of a typical commercial or hospital environment. |
| Surge
IEC 61000-4-5 |
±1kV Differential
±2kV Common |
±1kV Differential
±2kV Common |
Mains power quality should be that of a typical commercial or hospital environment. |
| Voltage dips, short | >95% Dip for 0.5 Cycle | >95% Dip for 0.5 Cycle | Mains power quality should be that of a typical |
| interruptions and voltage variations on power supply | 60% Dip for 5 Cycles
30% Dip for 25 Cycles |
60% Dip for 5 Cycles
30% Dip for 25 Cycles |
commercial or hospital environment. If the user of this device requires continued operation during power mains interruptions, it is |
| input lines | >95% Dip for 5 Seconds | >95% Dip for 5 Seconds | recommended that the device be powered from |
| IEC 61000-4-11 | an uninterruptible power supply or battery. | ||
| Power Frequency 50/60Hz Magnetic Field
IEC 61000-4-8 |
3A/m |
3A/m |
Power frequency magnetic fields should be
that of a typical location in a typical commercial or hospital environment. |
|
Conducted RF IEC 61000-4-6 |
3 Vrms from 150 kHz to 80 MHz |
V1 = 3 Vrms |
Portable and mobile RF communications equipment should be separated from the device by no less than the recommended separation distances calculated/listed below: D=(3.5/V1)√P |
|
Radiated RF IEC 61000-4-3 |
3 V/m 80 MHz to 2.5 GHz |
E1 = 3V/m |
D=(3.5/E1)√P 80 to 800 MHz
D=(7/E1)√P 800 MHz to 2.5 GHz Where P is the maximum power rating in watts and D is the recommended separation distance in meters. Field strengths from fixed transmitters, as determined by an electromagnetic site survey, should be less than the compliance levels (V1 and E1). Interference may occur in the vicinity of equipment containing a transmitter. |
| For transmitters rated at a maximum output power not listed above, the recommended separation distance D in meters
(m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people. |
|||
| Recommended Separation Distances Between Portable and Mobile RF Communications Equipment and this device. This device and system are NoT Life-Supporting | |||
| This device is intended for use in the electromagnetic environment in which radiated disturbances are controlled. The customer or user of this device can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF Communications Equipment and the device as recommended below, according to the maximum output power of the communications equipment. | |||
| Maximum Output Power (Watts) | Recommended Separation Distances for the device (meters) | ||
| 150 kHz to 80 MHz D=(1.1667)√P | 80 to 800MHz D=(1.1667)√P | 800 MHz to 2.5 GHz D=(2.3333)√P | |
| 0.01 | 0.11667 | 0.11667 | 0.23333 |
| 0.1 | 0.36894 | 0.36894 | 0.73785 |
| 1 | 1.1667 | 1.1667 | 2.3333 |
| 10 | 3.6894 | 3.6894 | 7.3785 |
| 100 | 11.667 | 11.667 | 23.333 |
| For transmitters rated at a maximum output power not listed above, the recommended separation distance D in meters
(m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people. |

[xyz-ips snippet=”download-snippet”]