DJO AIRCAST Cryo / Cuff IC User Manual
BEFORE USING THE DEVICE, PLEASE READ THE FOLLOWING INSTRUCTIONS COMPLETELY AND CAREFULLY. CORRECT APPLICATION IS VITAL TO THE PROPER FUNCTIONING OF THE DEVICE.
NOTICE: WHILE EVERY EFFORT HAS BEEN MADE IN STATE-OF-THE-ART TECHNIQUES TO OBTAIN THE MAXIMUM COMPATIBILITY OF FUNCTION, STRENGTH, DURABILITY AND COMFORT, THERE IS NO GUARANTEE THAT INJURY WILL BE PREVENTED THROUGH THE USE OF THIS PRODUCT.
INTENDED USER PROFILE
The intended user should be a licensed medical professional, the patient, the patient’s caretaker, or a family member providing assistance. The user should be able to:
- Read, understand and be physically capable to perform all the directions, warnings and cautions provided in the information for use.
OPERATING PRINCIPLE
The Cryo/Cuff IC Cold Therapy System provides cold and compression by exchanging chilled pressurized water between the cuff and the cooler intermittently.
INTENDED USE/INDICATIONS
The Cryo/Cuff™ IC Cold Therapy System provides intermittent compression to minimize swelling and pain.
The intended use of the AirCast Cryo/Cuff IC is for the temporary reduction of swelling and pain after surgery or injury. This is a non-sterile prescription device for single patient use to provide localized external application of cold therapy and intermittent compression. This device may not be used for any other purpose
CONTRAINDICATIONS
Raynaud’s or other vasospastic diseases; diabetics; sickle-cell anemia, arteriosclerosis, cryoglobulinemia, hypercoagulable clotting disorders or other peripheral vascular diseases causing ischemia or poor local circulation; compromised local circulation; local tissue infection; cold allergy, cold hypersensitivity, previous cold injury, and paroxysmal cold haemoglobinuria.
WARNING: Before using the Cryo/Cuff™ IC Cold Therapy System, read the enclosed instructions carefully and completely.
APPLICATION INFORMATION
Before applying the Cryo/Cuff, prepare the cooler (Figure 1)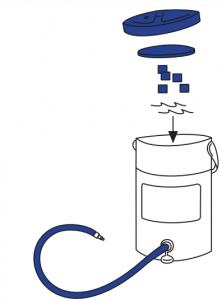
- Connect the blue tube to cooler.
- Add cold water to line inside cooler.
- Fill with ice.
- Lay insulation disk on top of ice. Attach cooler lid snugly. Allow five minutes with occasional shaking to chill water.
Always apply EMPTY Cryo/Cuff (Figure 2)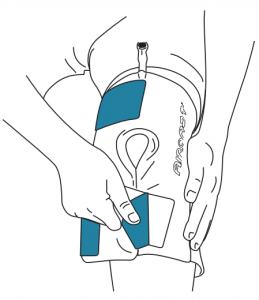
- Use only Aircast Cryo/Cuffs and ArcticFlow Cuffs with Aircast Cryo/Cuff IC Unit. Cuff is the Applied Part.
- Carefully and completely read instructions supplied with the specific Cryo/Cuff prior to application. Please follow the instructions below to continue cooler preparation.
Fill and pressurize Cryo/Cuff (Figure 3)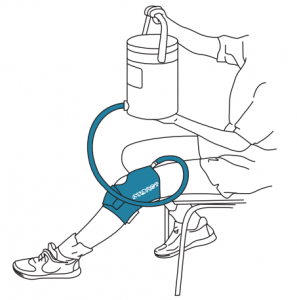
- To fill cuff: Connect blue tube to the cuff. Raise cooler no more than 15” (38cm) above the cuff for approximately 30 seconds while Cryo/Cuff fills.
- To correctly position cooler: Place cooler on stable surface.The cooler base should be even with the cuff.NOTE: To AVOID excessive pressure during use, DO NOT raise the cooler higher than 15” (38 cm) above the Cryo/ Cuff.
- To turn cooler on: Plug power supply into wall outlet and connect cord to lid.
- To turn off the equipment disconnect the power supply.
- To refill cooler: When Ice has melted , unplug the cooler and repeat items 1-5 under Step 1.
- For specific cuffs, please refer to instructions for installation of each cuff and follow procedure above.
WARNINGS AND CAUTIONS
- A licensed health care practitioner must select the length of use and frequency of use depending on individual patients needs. DO NOT use this device if a prescription has not been provided to you or if you do not understated the prescription as this may result in injury.
- Follow all precautions necessary to avoid electrical shock, fire, burns, or other personal injury from electrical power by using the device indoors, with dry hands, in a dry location. Keep all electrical connections away from water. Extreme care must be taken when using any cold therapy system. Any cold products may cause injuries, e.g. burns, blisters, frostbite, swelling, discoloration and skin or tissue necrosis if improperly used or monitored.
- Patient skin condition checks must occur every 1~2 hours on a routine basis. Caution must be taken by routinely monitoring the patient during use on children, elderly, incapacitated patients, severe cardiovascular diseases, those with decreased skin sensitivity or circulation due to poor nutrition, medications (e.g. beta blocker or epinephrine, narcotics), tobacco and alcohol use. If such monitoring is to occur outside the presence of the licensed healthcare professional, he or she must ensure that the patient or patient caretaker understands how such monitoring is to be conducted.
- If redness, itching, numbness, burning, pain or tingling increases, or unusual reactions like blisters, discoloration, swelling, welts, or other changes in skin appearance occur while using this product, consult your medical professional immediately.
- DANGER – Risk of explosion if used in the presence of flammable anesthetics or oxygen rich environment.
- To reduce the risk of electrical shock, do not disassemble unit. Return to manufacturer per Warranty.
- Do not allow fluid to come into contact with the power connection on lid.
- Only use power supply provided with unit. Failure to follow instructions could damage the pump, power supply or both.
- The product is not suitable for use in contact with foods and beverage.
- Dispose cooler and lid according to local, state and federal regulations.
- Keep power cord, hose, small parts and packaging materials away from small children and animals. These items pose a risk for suffocation or strangulation.
- It could be unsafe to use accessories, detachable parts and materials, or interconnect to other equipment not described in these instructions, or otherwise modify equipment.
- Care must be taken when operating this device adjacent to other equipment. Potentialelectromagnetic or other interferences could occur to this or other equipment. Try to minimize the interference by not using other electronic equipment in conjunction with this device.
- The power supply unit is the mains power disconnect. Do not position the equipment to make access to the disconnect difficult.
- It is recommended that at least 20 minutes of cessation should be encouraged between 2 hour long cryotherapy sessions.
- When applying the device wrap to the skin, apply a barrier (e.g. cloth or bandage) between the wrap and your skin, and do not wrap it around the area tightly. No part of the wrap should touch the skin for any period of time.
- The treatment site should be kept dry. Check for moisture on the barrier between your skin and cold pad. If moisture is present on the barrier, immediately discontinue use of this device.
- Ensure to speak with your healthcare provider prior to use if you suffer from Buerger’s disease, suffer from a condition that may decrease your skin sensation, such as peripheral neuropathy, sciatica, stroke or spinal cord injury, if you are taking medication that may negatively impact on peripheral vascular circulation (e.g. beta adrenergic blockers and local epinephrine) or, if you have a history of frostbite or adverse reactions to local cold application.
This device adheres to the following standards: IEC60601-1, IEC60601-1-2, IEC60601-1-6, IEC60601-1-11, IEC62366.
Cleaning and Storage
Possible Adverse Reactions
- Local skin irritation/ itching
- Burn
- Infection
- Frostbite
- Allergic Reaction
- Pain
- Transient Nerve Palsy
- Erythema
- Deep Vein Thrombosis
- Extensor Mechanism Disruption
- Necrosis
- Compartment Syndrome
- Blisters
- Neuropathy
- Cold Urticaria
- Slowed wound healing
- Perniosis
- Bradycardia
- Adverse changes to joint position sense, muscle strength, and neuromuscular performance
- Increased swelling
- Discolouration
- Welts
- Numbness
CLEANING AND STORAGE
Use mild detergent to clean cooler and lid as needed. Do not submerge lid under water. Unplug the power supply from the electrical outlet. TO AVOID DANGER OF ELECTRICAL SHOCK, DO NOT UNPLUGTHE POW ER SUPPLY WITH WET HANDS. No preventative maintenance is required other than cleaning. Periodically clean cuff, tube, and cooler using a few ounces of liquid soap added to hot water in cooler.Recycle soap/water mixture between cooler and cuff a few times, then repeat with warm water only. Rinse completely. Air dry after cleaning.
Nominal Operating EnvironmentAmbient Temperature: 5-40°CRelative Humidity: 15-90%Atmosphere Pressure: 700 h Pa – 1060 h Pa
Storage/Transportation EnvironmentAmbient Temperature: -20°C (-4°F) – 70°C (158°F)Relative Humidity: Up to 90%Atmosphere Pressure: 700 to 1060 h Pa (0.5 to 1.05 ATM)
Power Supply: AC/DC
- The power supply is only designed to connect to a 100-240 V main circuit.
- The system is a Class II device and does not rely on protective earth.
- To order a replacement power supply contact DJO Global Customer Support.
- Cryo/Cuff Domestic Power Supply: DJO P/N 13-4882-0-00000
- Cryo/Cuff International Power Supply: DJO P/N 13-2325
Malfunctions
- Device not powering on.
- Cuff not getting cold.
TROUBLESHOOTING
- Make sure unit has ice and water filled to the line indicated inside the cooler.
- Check power supply connections at wall and at the unit.
- Make sure to fill pad prior to operating unit as instructions indicate.
- Check pad connection; make sure to listen for a click when connecting the hose to the pad.
- Make sure unit and pad are level for optimal performance.
- This device may be susceptible to magnetic or electromagnetic interference. If this occurs, relocate or reposition the device. Increase the separation between the equipment. Plug the power supply into an outlet on a circuit different than other devices.
WARRANTY
DJO, LLC will repair or replace all or part of the unit and its accessories for material or workmanship defects for a period of 180 days from the date of sale. There are no serviceable parts in this product, return to manufacturer for repair or replacement.
S YMBOL EXPLANATION
| Attention / Read Manual | Class II Equipment | Type BF Equipment | Temperature Limits |
|
|
|
 |
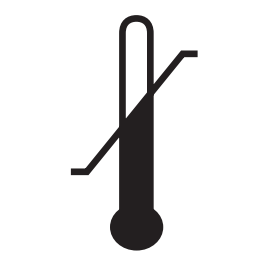 |
| Cold Temperature | Warning Sign | Manufacturer | Rx Only |
 |
 |
 |
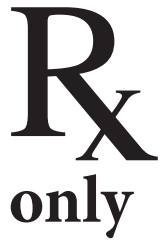 |
| Safety Mark | Humidity Limits | Atmospheric Pressure Limits | Degree of Ingress Protection |
ELECTROMAGNETIC COMPATIBILITY (EMC)
 |
 |
 |
 |
![]() This device must be separated from household waste and recycled as electronic waste
This device must be separated from household waste and recycled as electronic waste
Expected service life is min. 400 hours.
ELECTROMAGNETIC COMPATIBILITY (EMC )
Cryo Cuff IC has been tested and found to comply with the electromagnetic compatibility (EMC) limits for medical devices to IEC 60601-1-2. These limits are designed to provide reasonable protection against harmful interference in a typical medical installation .
CAUTION: Medical electrical equipment requires special precautions regarding EMC and must be installed and operated according to these instructions . It is possible that high levels of radiated or conducted radio-frequency electromagnetic interference (EMI) from portable and mobile RF communications equipment or other strong or nearby radio-frequency sources, could result in performance disruption of the system . Evidence of disruption may include equipment ceasing to operate, or other incorrect functioning. If this occurs, survey the site of disruption, and take the following actions to eliminate the source(s).
- Turn equipment in the vicinity off and on to isolate disruptive equipment .
- Relocate or reorient interfering equipment.
- Increase distance between interfering equipment and your system.
- Manage use of frequencies close to the system frequencies.
- Remove devices that are highly susceptible to EMI.
- Lower power from internal sources within the facility control (such as paging systems).
- Label devices susceptible to EMI.
- Educate clinical staff to recognize potential EMI-related problems.
- Eliminate or reduce EMI with technical solutions (such as shielding).
- Restrict use of personal communicators (cell phones, computers) in areas with devices susceptible to EMI .
- Share relevant EMI information with others, particularly when evaluating new equipment purchases which may generate EMI.
- Purchase medical devices that comply with IEC 60601-1-2 EMC Standards (3 V/meter EMI immunity, limit interference level to 0.0014 V/meter)
Electromagnetic Compatibility (EMC) Tables
RF EMISSIONS CLASS B
| Guidance and Manufacturer’s Declaration – Electromagnetic Immunity | |||
| Cryo Cuff IC is intended for use in the electromagnetic environment specified below. The customer or the user of Cryo Cuff IC should assure that it is used in such an environment. | |||
| Immunity Tests | IEC 60601
Test Level |
Compliance Level | Electromagnetic Environment
– Guidance |
| Electrostatic Discharge (ESD)
IEC 61000-4-2 |
±6 kV contact
±8 kV air |
±6 kV contact
±8 kV air |
Floors should be wood, concrete, or ceramic tile.
If floors are covered with synthetic material, the relative humidity should be at least 30%. |
| Electrical fast transient/ burst
IEC 61000-4-4 |
±2 kV for power supply lines
±1 kV for input/ output lines |
±2 kV for power supply lines
±1 kV for input/ output lines |
Mains power quality should be that of a typical commercial or hospital environment. |
|
Surge IEC 61000-4-5 |
±1 kV differential mode
±2 kV common mode |
±1 kV differential mode
±2 kV common mode |
Mains power quality should be that of a typical commercial or hospital environment. |
| Voltage dips, short
interruptions and voltage variations on power supply input lines IEC 61000-4-11 |
<5% UT (>95% dip in
UT ) for 0.5 cycle 40% UT (60% dip in UT ) for 5 cycles 70% UT (30% dip in UT ) for 25 cycles <5% UT (>95% dip in UT ) for 5 sec |
<5% UT (>95% dip in
UT ) for 0.5 cycle 40% UT (60% dip in UT ) for 5 cycles 70% UT (30% dip in UT ) for 25 cycles <5% UT (>95% dip in UT ) for 5 sec |
Mains power quality should be that of a typical commercial or hospital environment. If
the user of the Cold Therapy Unit requires continued operation during power mains interruptions, it is recommended that Cryo Cuff IC be powered from an uninterrupted power supply or a battery. |
| Power frequency (50/60Hz)
Magnetic fields IEC 61000-4-8 |
3 A/m | 30 A/m | Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. |
| NOTE: U is the a.c mains voltage prior to application of the test level. |
ELECTROMAGNETIC COMPATIBILITY (EMC)
| Guidance and Manufacturer’s Declaration – Electromagnetic Immunity | |||
| Immunity Tests | IEC 60601
Test Level |
Compliance Level | Electromagnetic Environment – Guidance |
| Cryo Cuff IC is intended for use in the electromagnetic environment specified below. The customer or the user of Cryo Cuff IC should assure that it is used in such an environment. | |||
| Conducted RF IEC 61000-4-6 | 3 Vrms
150 kHz to 80 MHz |
3 V | Portable and mobile RF communications equipment should be used no closer to any part of the Cold Therapy Unit, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance: d = [3.5/V1]√P 150 KHz to 80 MHz d = [3.5/E1]√P 80 MHz to 800 MHz d = [7/E1]√P 800 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey a, should be less than the compliance level in each frequency range b. Interference may occur in the vicinity of equipment marked with the following symbol:
|
| Radiated RF IEC 61000-4-3 | 3 V/m
80 MHz to 2.5 GHz |
3 V/m | |
| NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which Cryo Cuff IC is used exceeds the applicable RF compliance level above, Cryo Cuff IC should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Cold Therapy Unit. bOver the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1] V/m. |
| Guidance and Manufacturer’s Declaration – Electromagnetic Immunity | |||
| Cryo Cuff IC is intended for use in the electromagnetic environment specified below. The customer or the user of Cryo Cuff IC should assure that it is used in such an environment. | |||
| Immunity Tests | IEC 60601
Test Level |
Compliance Level | Electromagnetic Environment
– Guidance |
| Electrostatic Discharge (ESD)
IEC 61000-4-2 |
±6 kV contact
±8 kV air |
±6 kV contact
±8 kV air |
Floors should be wood, concrete, or ceramic tile.
If floors are covered with synthetic material, the relative humidity should be at least 30%. |
| Electrical fast transient/ burst
IEC 61000-4-4 |
±2 kV for power supply lines
±1 kV for input/ output lines |
±2 kV for power supply lines
±1 kV for input/ output lines |
Mains power quality should be that of a typical commercial or hospital environment. |
| Surge
IEC 61000-4-5 |
±1 kV differential mode
±2 kV common mode |
±1 kV differential mode
±2 kV common mode |
Mains power quality should be that of a typical commercial or hospital environment. |
| Voltage dips, short
interruptions and voltage variations on power supply input lines IEC 61000-4-11 |
<5% UT (>95% dip in
UT ) for 0.5 cycle 40% UT (60% dip in UT ) for 5 cycles 70% UT (30% dip in UT ) for 25 cycles <5% UT (>95% dip in UT ) for 5 sec |
<5% UT (>95% dip in
UT ) for 0.5 cycle 40% UT (60% dip in UT ) for 5 cycles 70% UT (30% dip in UT ) for 25 cycles <5% UT (>95% dip in UT ) for 5 sec |
Mains power quality should be that of a typical commercial or hospital environment. If
the user of the Cold Therapy Unit requires continued operation during power mains interruptions, it is recommended that Cryo Cuff IC be powered from an uninterrupted power supply or a battery. |
| Power frequency (50/60Hz)
Magnetic fields IEC 61000-4-8 |
3 A/m | 30 A/m | Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. |
| NOTE: U is the a.c mains voltage prior to application of the test level. |
| Guidance and Manufacturer’s Declaration – Electromagnetic Immunity | |||
| Immunity Tests | IEC 60601
Test Level |
Compliance Level | Electromagnetic Environment – Guidance |
| Cryo Cuff IC is intended for use in the electromagnetic environment specified below. The customer or the user of Cryo Cuff IC should assure that it is used in such an environment. | |||
| Conducted RF IEC 61000-4-6 | 3 Vrms
150 kHz to 80 MHz |
3 V | Portable and mobile RF communications equipment should be used no closer to any part of the Cold Therapy Unit, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance: d = [3.5/V1]√P 150 KHz to 80 MHz d = [3.5/E1]√P 80 MHz to 800 MHz d = [7/E1]√P 800 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey a, should be less than the compliance level in each frequency range b. Interference may occur in the vicinity of equipment marked with the following symbol:
|
| Radiated RF IEC 61000-4-3 | 3 V/m
80 MHz to 2.5 GHz |
3 V/m | |
| NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a.Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which Cryo Cuff IC is used exceeds the applicable RF compliance level above, Cryo Cuff IC should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Cold Therapy Unit. b. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1] V/m. |
| Recommended separation distances between portable and mobile RF communications equipment and the Cold Therapy Unit | |||
| Cryo Cuff IC is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of Cryo Cuff IC can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and Cryo Cuff IC as recommended below, according to the maximum output power of the communications equipment. | |||
| Rated Maximum Output Power of Transmitter | SEPARATION DISTANCE ACCORDING TO FREQUENCY OF TRANSMITTER
m |
||
| W | 150 kHz to 80 MHz d = [3.5/V1]√P | 80 MHz to 800 MHz d = [3.5/E1]√P | 800 MHz to 2.5 GHz d = [7/E1]√P |
| 0.01 | 0.12 | 0.12 | 0.23 |
| 0.1 | 0.38 | 0.38 | 0.73 |
| 1 | 1..2 | 1.2 | 2.3 |
| 10 | 3.8 | 3.8 | 7.3 |
| 100 | 12 | 12 | 23 |
| For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people. |
ACCESSORY LIST
| Aircast ®CryoCuff™ Shoulder | ||
| 12A01 | Cuff Only 32” – 48’” | |
| 12B01 | Low Profile Cuff Only 32” – 48” | |
| A1220 | Strap Only 32” – 48” | |
| 1220AXL | Cuff w/X-Long
42” – 54” Strap and Cooler |
|
| 12AXL01 | Cuff Only
42” – 54” w/X-Long Strap |
|
| A1220XL | X-Long Strap Only 42” – 54” | |
| THIGH | ||
| 13A01 | Cuff Only, L 17” – 23” | |
| 13B01 | Cuff Only, XL 20” – 27” | |
| 13C01 | Cuff Only, Universal 14” – 20” | |
| Back/HIP/Rib/CryoCuff™ | ||
| 14A01 | Cuff Only | |
| Elbow | ||
| 15A01 | Cuff Only | |
| Hand or Wrist | ||
| 16A01 | Cuff Only | |
| Knee CryoCuff™ | ||
| 11C01 | Cuff Only 10” – 19” S | |
| 11A01 | Cuff Only 18” – 23” M | |
| 11B01 | Cuff Only. 20” – 31” L | |
| 11D01 | Cuff, Lt
18” – 23” M |
|
| 11E01 | Cuff, Lt 20” – 31” L | |
| 11P01 | Knee cuff, Pediatric | |
| Ankle CryoCuff™ | ||
| 10A01 | Cuff only | |
| 10A01V1356 | Ankle/Calf/Leg Cry/ Cuff | |
| 10P01 | Cuff Only, Pediatric | |
| Foot CryoCuff™ | ||
| C10C | Cuff and Cooler 9” – 13” M | |
| 10C01 | Cuff Only 9” – 13” M | |
| C10B | Cuff and Cooler. 10” – 17” L | |
| 10B01 | Cuff Only 10” – 17” L | |
| 10P01 | Pediatric Ankle Cuff Only |
PRODUCT SUPPORT
For product support call +1-888-405-3251 or +1-760-727-1280.
Contact manufacturer if assistance is needed in setting up, using or maintaining the equipment or to report unexpected operation or events.
Please report any malfunctions or serious adverse events occuring due to usage of this device to DJO and your local competent authority.

[xyz-ips snippet=”download-snippet”]