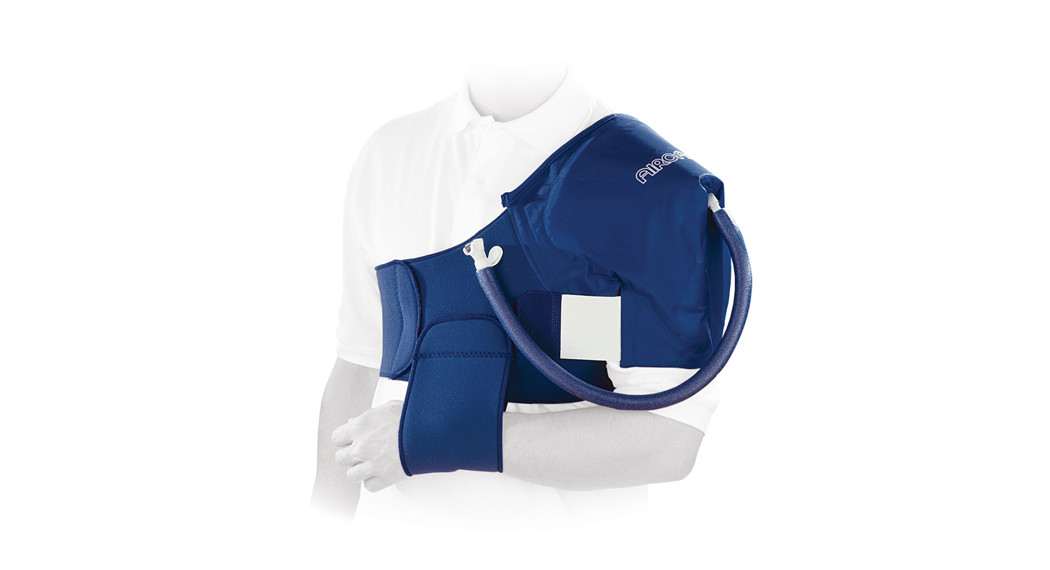
SHOULDERCRYO/CUFFCRYO/CUFF PARA HOMBRO
BEFORE USING THE DEVICE, PLEASE READ THE FOLLOWING INSTRUCTIONS COMPLETELY AND CAREFULLY. CORRECT APPLICATION IS VITAL TO THE PROPER FUNCTIONING OF THE DEVICE.IF YOU ARE USING THE CRYO/CUFF WITH THE AIRCRAFT CRYO/CUFF IC UNIT BE SURE YOU READ AND UNDERSTAND ALL INSTRUCTIONS LOCATED IN THE AIRCRAFT CRYO/CUFF IC INSTRUCTIONS FOR USE.INTENDED USER PROFILE: The intended user should be a licensed medical professional, the patient, the patient’s caretaker, or a family member providing assistance. User should be able to:
- Read and understand the directions, warnings and cautions.
OPERATING PRINCIPLE: The AirCast Cryo/Cuff provides cold therapy and compression.INTENDED USE/INDICATIONS: The Aircast Cryo/Cuff combines focal compression with cold to minimize swelling and pain.FREQUENCY OF USE: The length of use and frequency of use of the Cryo/Cuff are determined by the healthcare professional depending on the individual patient’s needs. This device can be cold enough to cause serious injury be sure to check skin frequently.CONTRAINDICATIONS: Cryotherapy should not be used on persons with Raynaud’s or another vasospastic disease, cold hypersensitivity, decreased skin sensitivity, compromised local circulation, diabetics, sickle-cell anemia, arteriosclerosis, cryoglobulinemia, hypercoagulable clotting disorders or other peripheral vascular diseases causing ischemia or poor local circulation, local tissue infection, cold allergy, cold hypersensitivity, previous cold injury and paroxysmal cold hemoglobinuria.Do not use if you are allergic to any of the materials contained within this product.WARNINGS AND CAUTIONS:
- This device can be cold enough to cause serious injury, including tissue necrosis.You must be able to check your skin condition under the Cuff (DO NOT use if you cannot check your skin condition frequently). People are sensitive to cold in diverseways and may react differently to cold treatment.
- Check for increased pain, burning, numbness, tingling, increased redness, discoloration, itching, increased swelling, blisters, welts, irritation, or other changes in skin condition under the Cuff or around the treatment area. If you experience any of these conditions, immediately discontinue use of the device and contact your physician. • Patient skin condition checks must occur every 1~2 hours on a routine basis. Caution must be taken by routinely monitoring the patient during use on children, elderly, incapacitated patients, severe cardiovascular diseases, those with decreased skin sensitivity or circulation due to poor nutrition, medications (e.g. beta-blocker or epinephrine, narcotics), tobacco and alcohol use. If such monitoring is to occur outside the presence of the licensed healthcare professional, he or she must ensure that the patient or patient caretaker understands how such monitoring is to be conducted.
- It is recommended that at least 20 minutes of cessation should be encouraged between 2-hour long cryotherapy sessions.
- When applying the device wrap to the skin, apply a barrier (e.g. cloth or bandage) between the wrap and your skin, and do not wrap it around the area tightly. No part of the wrap should touch the skin for any period of time.
- Check for moisture. If moisture is present on the Cuff, immediately discontinue the use of the device.
- Do not use an elastic wrap in conjunction with the Cryo/Cuff.
- Dressings used under the Cryo/Cuff should be applied lightly.
- When filling the Cryo/Cuff, do not raise the cooler higher than 15 inches (38 cm) above the cuff to avoid excessive pressure.
- Reduce pressure with any sense of discomfort, numbness, or tingling of the limb.
- The Cryo/Cuff is designed for single patient use only and may be used on the same patient for the length of treatment.
- The device should be cleaned if it is used on the same patient for an extended period of time (see “Cleaning Instructions” for cleaning information).
- Keep small parts and packaging materials away from small children and animals. These items pose a risk for suffocation or strangulation.
- It could be unsafe to use accessories, detachable parts and materials, or interconnect to other equipment not described in these instructions, or otherwise modified equipment.
APPLICATION INFORMATION:
- PREPARE COOLER- Connect blue tube to cooler. Add water to line inside cooler, then add ice to the top of cooler. Lay the insulation disk on top of the ice. Attach cooler lid snugly. Allow 5 minutes with occasional shaking to chill water.
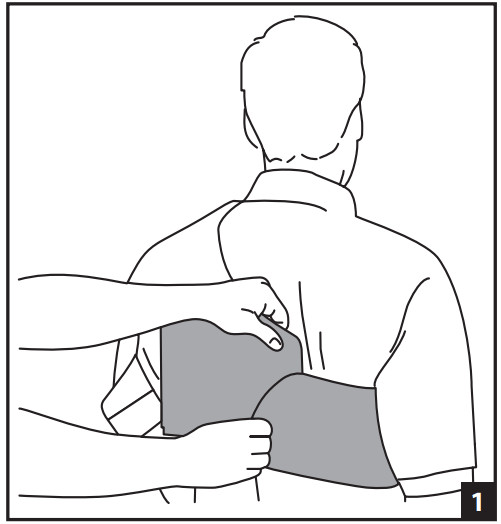
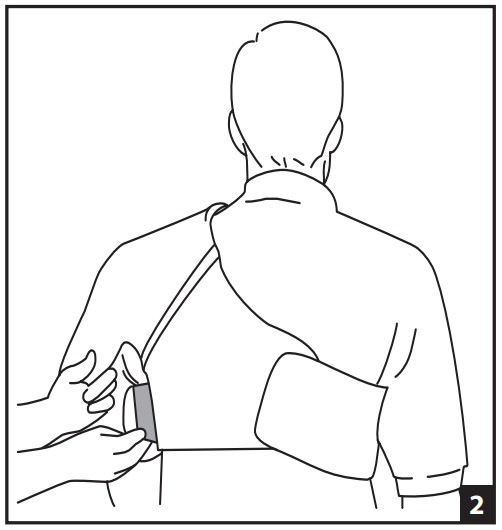
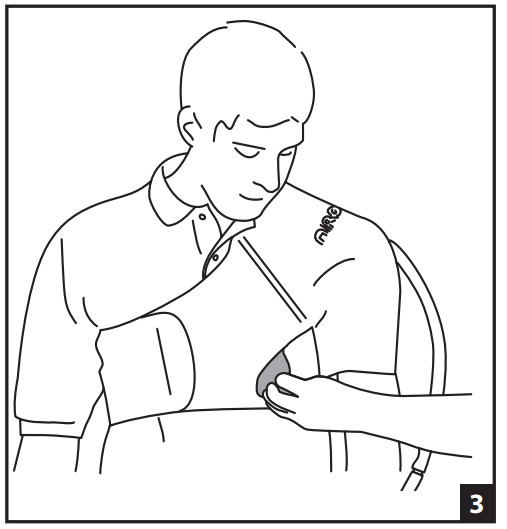
- APPLY THE EMPTY CUFF- At first, it is helpful to have another person assist with the application of the cuff. Attach the main straps around the torso securing in front and back (Fig. 1). Secure underarm strap (Fig. 2). Secure front and back elastic straps (Fig. 3). The elastic straps can be opened during shoulder exercises.
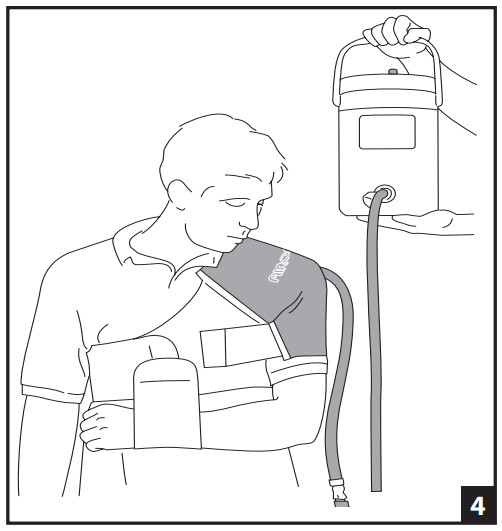
- FILL CUFF- Connect blue tube to the cuff, listen for a click/snap. When using the Gravity Cryo/Cuff (non-motorized), open-air vent on cooler lid and raise cooler so that bottom of the cooler is even with the top of the cuff (Fig. 4). (If the cooler is raised more than 5 inches (13 cm) above the top of the cuff, the cuff will increase in volume and weight.) Hold raised cooler for about 1 minute while cuff fills. Close air vent on the cooler lid. The cooler can now be disconnected from cuff by pressing the metal tab on the quick-disconnect while the cooler is raised.
- RECHILL WATER- Reconnect the blue tube to the cuff, lower the cooler below cuff, and the warmed water will drain from the cuff into the cooler. Allow a minute or two for the water to mix with the ice and rechill, then raise the cooler and repeat the filling process (see step 3).CLEANING INSTRUCTIONS: After use, completely drain water from the cuff, tube, and cooler. (To drain tube, elevate tube while pressing tip of quick-disconnect). Store cooler with top off to allow drying. Periodically clean cuff, tube, and cooler using a few ounces of liquid soap added to hot water in cooler. Recycle soap/water mixture between cooler and cuff a few times, then repeat with warm water only. Rinse completely.
MATERIAL CONTENTS: Polyurethane Nylon, Nylon, Polyether foam.WARRANTY: DJO, LLC will repair or replace all or part of the unit and its accessories for material or workmanship defects for a period of six months from the date of sale.To the extent the terms of this warranty are inconsistent with local regulations, the provisions of such local regulations will apply.EXPLANATION OF SYMBOLS:
![]() Warnings
Warnings
RX ONLY.FOR SINGLE PATIENT USE ONLY.![]() NOT MADE WITH NATURAL RUBBER LATEX.
NOT MADE WITH NATURAL RUBBER LATEX.
NOTICE: WHILE EVERY EFFORT HAS BEEN MADE IN STATE-OF-THE-ART TECHNIQUES TO OBTAIN THE MAXIMUM COMPATIBILITY OF FUNCTION, STRENGTH, DURABILITY AND COMFORT, THERE IS NO GUARANTEE THAT INJURY WILL BE PREVENTED THROUGH THE USE OF THIS PRODUCT.
![]()
MDSS GmbHSchiffgraben 4130175 Hannover, Germany©2021 DJO, LLC

DJO, LLC1430 Decision StreetVista, CA 92081-8553U.S.A.12A100 REV AF – 2021-03-15
[xyz-ips snippet=”download-snippet”]