![]()
![]()
![]()
![]()










BEFORE USING THE DEVICE, PLEASE READ THE FOLLOWING INSTRUCTIONS COMPLETELY AND CAREFULLY. CORRECT APPLICATION IS VITAL TO THE PROPER FUNCTIONING OF THE DEVICE.
INTENDED USER PROFILE: The intended user should be a licensed medical professional, the patient or the patient’s caregiver. The user should be able to read, understand and be physically capable of performing the directions, warnings and cautions in the information for use.
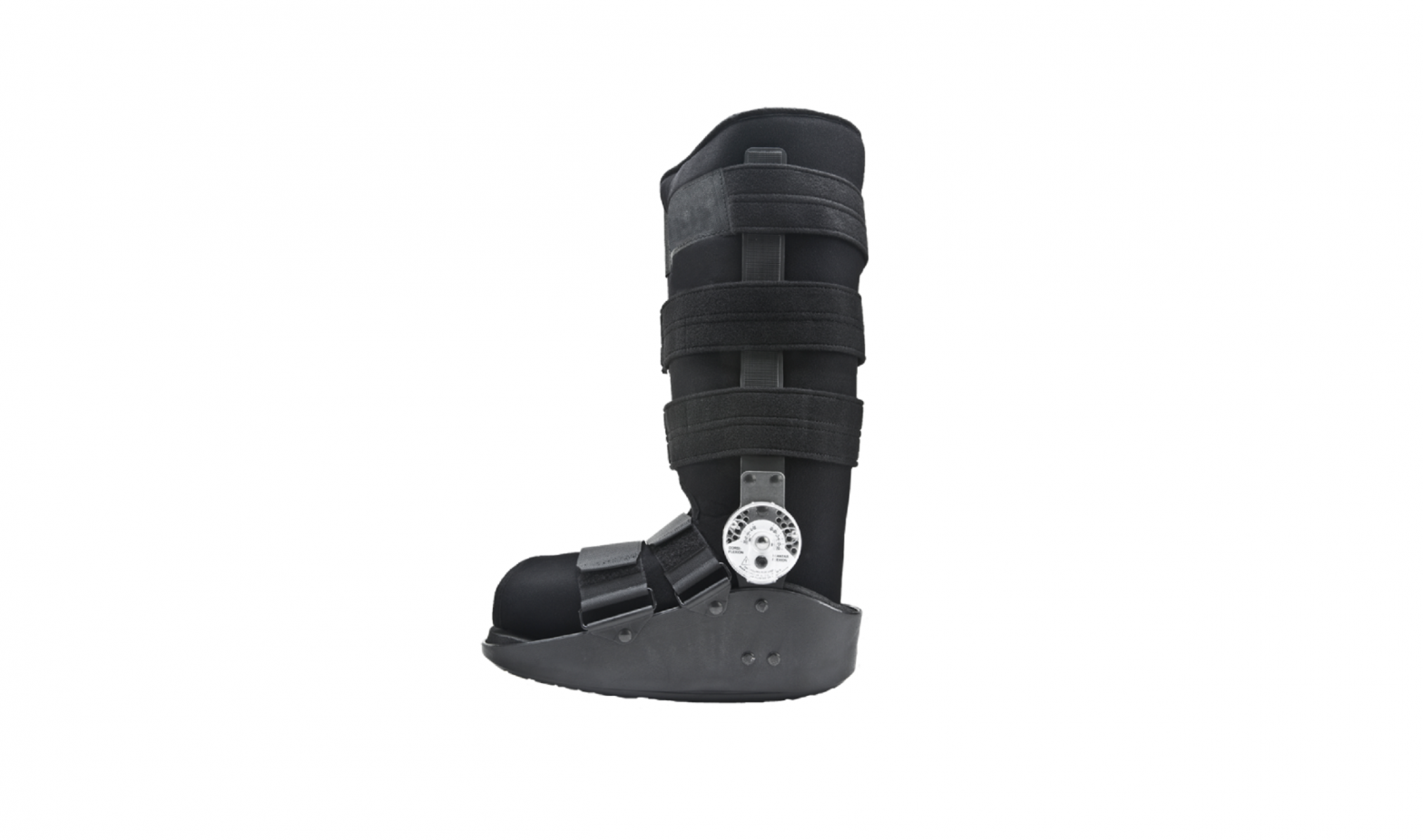
INTENDED USE/INDICATIONS: The Procare MaxTrax Walker is designed to provide support and immobilization to the lower leg, ankle and foot. It may be suitable for the treatment of stress fractures of the lower limb, stable ankle and/or foot fractures, acute lateral ankle sprains and Achilles tendon repair. Providing immobilization or controlled movement of the limb or body segment.
CONTRAINDICATIONS: This device is contraindicated for unstable fractures of the lower leg, ankle and foot.
PRESCRIBING INFORMATION: Note: Due to the molded outer sole, the orthopedic walker may be slightly higher than a normal street shoe, therefore an athletic shoe or a street shoe with a ½” heel, may be worn if necessary for patient comfort. A heel lift also may be used in the shoe worn on the non-affected foot.
WARNINGS AND PRECAUTIONS:
- This device is to be used under the supervision of a healthcare professional. The determination of when to apply the brace and the frequency and duration of use should be strictly at the discretion of the treating physician. Always consult with your physician before making changes to the brace.
- Application of this device is recommended only when the fracture is demonstrably stable and there area acceptable limits of angular and rotational deformity.
- Do not use this device on patient’s incapable of communicating physical discomfort.
- Check your skin frequently for “hot spots” and skin irritation.
- Do not use this device if you cannot feel sensations whether due to post-op anaesthesia, nerve, skin or other conditions.
- Use caution when walking on slippery or wet surfaces to avoid injury.
- Do not use over open wounds.
- Do not over tighten straps. This may result in reduction of blood flow or sensation.
- Do not modify the device or use the device other than intended.
- If you develop an allergic reaction and/or experience itchy, red skin after coming into contact with any part of this device, please stop using it and contact your healthcare professional immediately.
- Do not use this device if it is damaged and/or the packaging has been opened.
- If pain, swelling, changes in sensation or other unusual reactions occur while using this product, consult your medical professional immediately.
NOTE: Contact manufacturer and competent authority in case of a serious incident arising due to usage of this device.
APPLICATION INFORMATION: For first time application, loosen all straps and remove liner from boot.
- Two removable foam ankle pads are provided for patient comfort.
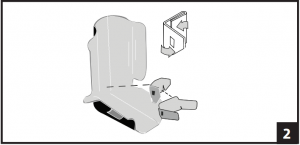
- If pads require adjustment, remove both pads from liner and separate. Smaller pad should be placed in liner first. At V-cutouts, fold side panels toward center. Ensure the three (3) Velcro® strips are centered on each panel. (Fig. 1) Folded pad should be centered on back of liner with V-cutouts positioned at base of heel. Open side panels of pad and secure on either side of liner Note: Pad should fit around ankle area when applied correctly. Large pad may be applied directly to liner or layered on top of small pad as needed. Application is same as small pad. (Fig. 2)
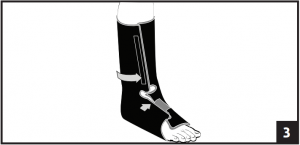
- Place foot in liner and secure with contact closure. Make sure the heel fits snugly into the posterior portion of the liner. Secure the forefoot flap on the liner first followed by the lower leg portion. Make sure liner fits snug top to bottom. (Fig. 3)
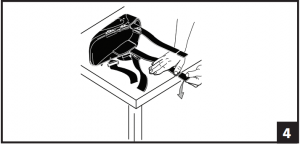
- Both uprights may be shortened by 2” to accommodate various patient heights. To Breakdown Uprights: Secure uprights on edge of table or hard surface. Firmly push down on breakdown portion of bar. Soft Velcro® strips are included at top of each liner. Following breakdown of bars, the strips should be applied to top of uprights or liner may be folded over uprights if necessary for added comfort and fit. (Fig. 4)
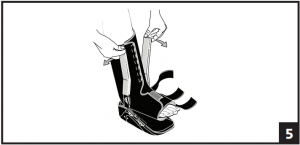
- Spread the uprights using both hands and step into boot, aligning uprights with midline of the ankle. Remove plastic sheaths on uprights and secure liner. The uprights may be bent to accommodate any leg contour. (Fig. 5)
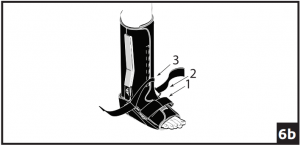
- The two straps at the ankle joint may be adapted to accommodate individual patient needs. Prior to securing bootstraps, determine desired ankle strapping method. Ankle Cross Strap Conversion: 6a) Strap 3 is removable and may be secured by feeding through d-ring on opposing upright. 6b) If Cross Strapping is desired, feed Strap 3 through opposing forefoot d-ring. Cross Strap 2 over Strap 3, feed through d-ring on opposing upright and secure. Secure all other bootstraps, starting at the toes and working up the leg.
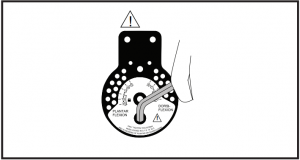
Instructions for Range-of-Motion hinge adjustment: Slide open cap cover. Turn hinge cap cover to expose pins. Release pins, select correct settings and reinsert pins. Close cap cover. To lock uprights in fixed 90° angle, set both pin settings at 0°. To lock in 7.5°, 15°, 22.5° or 30° of plantar or dorsiflexion, set one pin at desired setting in location marked “lock” and second pin in corresponding (same number) setting in the open opposing position.


CLEANING INSTRUCTIONS: To clean liner, hand wash in cold water using mild soap. Air dry. Note: If not rinsed thoroughly, residual soap may cause irritation and deteriorate material.
WARRANTY: DJO, LLC will repair or replace all or part of the unit and its accessories for material or workmanship defects for a period of six months from the date of sale. To the extent the terms of this warranty are inconsistent with local regulations, the provisions of such local regulations will apply.
PATENT PENDING.

NOTICE: WHILE EVERY EFFORT HAS BEEN MADE IN STATE-OF-THE-ART TECHNIQUES TO OBTAIN THE MAXIMUM COMPATIBILITY OF FUNCTION, STRENGTH, DURABILITY AND COMFORT, THERE IS NO GUARANTEE THAT INJURY WILL BE PREVENTED THROUGH THE USE OF THIS PRODUCT.















MDSS GmbH DJO, LLCSchiffgraben 41 15919 Sea Otter Place Suite 20030175 Hannover, Germany Carlsbad, CA 92010 U.S.A.
©2021 DJO, LLC 13-2966-0-00000 REV F – 2021-1-15
[xyz-ips snippet=”download-snippet”]