DJO Twist Subtalar Implant System User Guide
The Twist Subtalar Implant is a titanium alloy capsule shaped implant intended to provide consistent correction in subtalar joint arthroereisis.
SUBTALAR SOLUTIONS

- Soft, double lead, inverted thread profile intended to provide significant load bearing surface area across the implant
- Radiused trailing and leading edges to mitigate potential lateral pressure points
- Trestle structure with up to 52 microchannels intended to accelerate tissue integration to mitigate implant migration
- Color coded, radio-opaque, smooth trials to preserve joint tissue while sizing
TWIST SUBTALAR IMPLANT SYSTEM
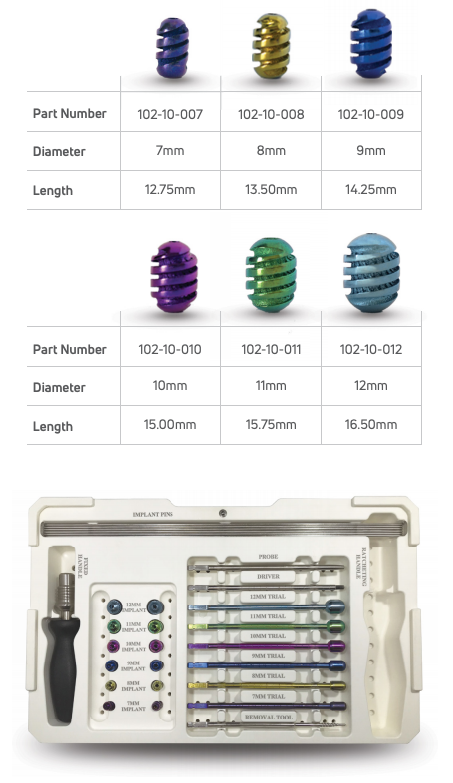
All associated instrumentation included in a single system
SURGICAL TECHNIQUE
STEP 1: Make a 2-3cm incision on the lateral aspect of the foot over the sinus tarsi along the relaxed skin tension lines.Avoid the intermediate dorsal cutaneous nerves that should course superior to the incision and sural nerve, which should course inferior to the incision.
STEP 2: Identify the deep facia and bluntly dissect allowing entrance into the lateral sinus tarsi.
STEP 3: Insert the guide pin into the sinus tarsi from lateral to medial until tenting is noted anterior and slightly inferior to the medial maleollus.
STEP 4: Introduce the cannulated probe over the guide pin and into the sinus tarsi with a gentle twisting motion to slightly dilate the tarsal canal.
STEP 5: Remove the cannulated probe and leave the guide pin in place.
STEP 6: Choose the appropriate trial based on the size and anatomy of the patient. The use of intra-operative AP and lateral view imaging is recommended to evaluate the placement of the trial. Introduce the selected cannulated trial over the guide pin into the sinus tarsi from lateral to medial until the leading edge of the trial is 1/3 to half way across the subtalar joint. The leading edge of the trial should not cross the longitudinal bisection of the talus and the trailing edge of the implant should be more than 5mm medial to the lateral wall of the calcaneous. The appropriate trial size should limit abnormal calcaneal eversion and will allow approximately 2-4 degrees of subtalar joint eversion. Once the appropriate size trial is determined, make note of the depth measurement on the calibrated section of the trial at the skin line and remove the trial from the joint while leaving the guide pin in place.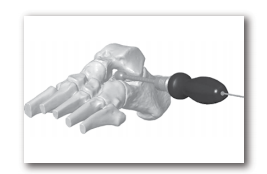
STEP 7: Place the equivalent size implant onto the insertion tool and introduce over the guide pin and thread into the joint with a clockwise rotation to the predetermined length noted from the depth measurement determined from the trial until clinical correction is noted. Intraoperative imaging in the AP and lateral view should be used to verify the final placement of the implant. The leading edge of the implant should be 1/3 to half way across the subtalar joint and the leading edge of the implant should not cross the longitudinal bisection of the talus while the trailing edge should be more than 5mm medial to the lateral wall of the calcaneous.
STEP 8: Once the final placement of the implant has been achieved, assess the range of motion of the subtalar joint. A significant reduction of excess subtalar joint pronation should now be achieved.
STEP 9: Remove the insertion tool and the guide pin.
STEP 10: Irrigate then close the deep tissue, fascia, subcutaneous tissue, and skin layers. Place the foot in a mild compressive dressing.
POST-OPERATIVE CARE
Assuming no adjunctive procedures were performed, a protective, weight bearing, below the knee walking cast or boot for 2-4 weeks is used. A gradual return to limited activity in 4-6 weeks is permitted as tolerated.
IMPLANT REMOVAL
In the event the implant needs to be removed, the threaded removal tool is inserted into the proximal end of the implant and turned in a counterclockwise motion to engage the reverse threads in the cannulation of the implant to back out and remove the implant.
Individual results may vary. DJO, LLC is a manufacturer of orthopedic implants and does not practice medicine. Only an orthopedic, or foot and ankle surgeon can determine what treatment is appropriate. The contents of this document do not constitute medical, legal, or any other type of professional advice. This material is intended for the sole use and benefit of the DJO, LLC sales force and physicians. It is not to be redistributed, duplicated, or disclosed without the express written consent of DJO, LLC. For more information on risks, warnings, and possible adverse side effects refer to the Instructions for Use provided with the device.
Certain system features are covered under U.S. Patent No. 8,545,572.FDA cleared 510(k) K103183.Trilliant products are made in the U.S.A.

T 800.495.2919 F 877.778.3864727 North Shepherd Drive, Suite 100 I Houston, TX 77007 I U.S.A.djoglobal.com
Copyright © 2021 by DJO, LLC900-00-011 Rev J
References
[xyz-ips snippet=”download-snippet”]

