GIMA Walking Frame With 2 Wheels User Manual

All serious accidents concerning the medical device supplied by us must be reported to the manufacturer and competent authority of the member state where your registered office is located.
INTRODUCTIONThis manual provides the instructons for a correct use of our patient aid or an assistant / carer.INTENDED USEThe walking frame is designed to be used inside buildings by patients with an impairment to legs. The devices are intended to provide a support to the body. This aid allow patient mobility making it easy to walk. They must be used withtwo arms in order to guarantee safe operation.WARNING! The walking frame is built for indoor use and only on flatsurfaces. If it is necessary to employ the devices outside we advise to follow the road rules like any pedestrian.MAINTENANCE The walking frame do not require special care for their ordinary maintenance. We advise to regularly perform the following checks: – verify the reliability of different components- check the correct fixing of screws and nuts- test moving parts, apply lubricant if needed – clean parts with mild detergents (do not use corrosive or harmful chemicals), put it in dry and ventilated environment.WARNING! Do not use the equipment in case it is damaged or any component is missing. Do not use the patient aids for purposes not mentioned in this user manual. Do not cross obstacles or use the patient aids on uneven surfaces, or wet and slippery. The use of the device is not recommended for people with poor functionality in the hands and arms.
Max load 100kg
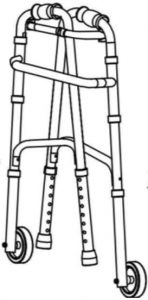
fig. 1Step 1: take out walker from package, open frame as pic 1, the frame is fixed when you heard a locking noise
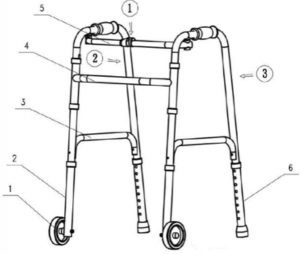
fig 2Step 2: to close the frame, press the button as 1 shows on pic 2, thenfold left and right frame as 2 & 3 show.
|
N° |
Part name |
QTY |
|
1 |
Wheel |
2 |
|
2 |
Leg tube |
2 |
|
3 |
Connected frame |
2 |
|
4 |
Cross bar |
1 |
|
5 |
Stretch frame |
1 |
|
6 |
Adjustable leg tube |
2 |
 |
Manufacturer |
 |
Keep in a cool, dry place |
 |
Consult instructions for use |  |
Keep away from sunlight |
 |
Product code |  |
Medical Device compliant with Regulation (EU) 2017/745 |
 |
Lot number |
 |
Caution: read instructions (warnings) carefully |
 |
Medical Device |
References
[xyz-ips snippet=”download-snippet”]

