For Rapid Detection of SARS-COV-2
INTENDED USE
The Coronavirus Ag Rapid Test Cassette (Swab) is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) swab specimens directly from individuals who are suspected of COVID-19 by their healthcare provider. It is intended to aid in the rapid diagnosis of SARS-CoV-2 infections. The Coronavirus Ag Rapid Test Cassette (Swab) does not differentiate between SARS-CoV and SARS-CoV-2.
SUMMARY AND EXPLANATION
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue, and dry cough. Nasal congestion, runny nose, sore throat, myalgia, and diarrhea are found in a few cases.
This test is for detection of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively.
PRINCIPLE OF THE TEST
The Coronavirus Ag Rapid Test Cassette (Swab) is an immunochromatographic membrane assay that uses highly sensitive monoclonal antibodies to detect nucleocapsid protein from SARS-CoV-2 in direct nasopharyngeal (NP) swab. The test strip is composed of the following parts: namely sample pad, reagent pad, reaction membrane, and absorbing pad. The reagent pad contains the colloidal-gold conjugated with the monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2; the reaction membrane contains the secondary antibodies for nucleocapsid protein of SARS-CoV-2. The whole strip is fixed inside a plastic device. When the sample is added into the sample well, conjugates dried in the reagent pad are dissolved and migrate along with the sample. If SARS-CoV-2 nucleocapsid antigen is present in the sample, a complex forms between the anti- SARS-2 conjugate and the virus will be captured by the specific anti-SARS-2 monoclonal antibodies coated on the test line region (T). Absence of the test line (T) suggests a negative result. To serve as a procedural control, a red line will alwaysappear in the control line region (C) indicating that proper volume of sample has been added and membrane wicking has occurred.
MATERIALS PROVIDED
- 20 Test Cassettes
- 2 Extraction Buffer Vials
- 20 Sterile Swabs
- 20 Extraction Tubes and Tips
- 1 Workstation
- 1 Package Insert
MATERIALS REQUIRED BUT NOT PROVIDED
- Clock, timer, or stopwatch
WARNINGS AND PRECAUTIONS
- For in vitro diagnostic use only.
- The test device should remain in the sealed pouch until use.
- Do not use kit past its expiration date.
- Swabs, tubes, and test devices are for single use only.
- Solutions that contain sodium azide may react explosively with lead or copper plumbing. Use large quantities of water to flush discarded solutions down a sink.
- Do not interchange or mix components from different kit lots.
- Testing should only be performed using the swabs provided within the kit.
- To obtain accurate results, do not use visually bloody or overly viscous samples.
- Wear suitable protective equipment gloves when handling specimen and the contents of this kit.
- Inadequate or inappropriate specimen collection and storage can adversely affect results.
- Humidity and temperature can adversely affect results.
- Dispose of test device and materials as biohazardous waste in accordance with federal, state, and local requirements
STORAGE AND STABILITY
- The kit can be stored at room temperature or refrigerated (2-30°C).
- Do not freeze any of the test kit components.
- Do not use test device and reagents after expiration date.
- Test devices that have been outside of the sealed pouch for more than 1 hour should be discarded.
- Close the kit box and secure its contents when not in use.
SPECIMEN COLLECTION
- Using the sterile nasopharyngeal swab provided in the kit, carefully insert the swab in the patient’s nostril.
- Swab over the surface of the posterior nasopharynx and rotate the swab several times.
- Withdraw the swab from the nasal cavity.

SAMPLE PREPARATION PROCEDURE
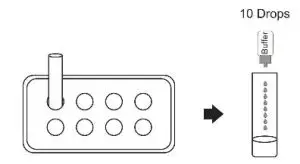
- Insert the test extraction tube into the workstation provided in the kit. Make sure that the tube is standing upright and reaches the bottom of the workstation.
- Add 0.3 mL (approximately 10 drops) of the sample extraction buffer into the extraction tube.

- Insert the swab into the extraction tube which contains 0.3 mL of the extraction buffer.
- Roll the swab at least 6 times while pressing the head against the bottom and side of the extraction tube.
- Leave the swab in the extraction tube for 1 minute.
- Squeeze the tube several times from the outside to immerse the swab. Remove the swab.

SPECIMEN TRANSPORT AND STORAGE
Do not return the nasopharyngeal swab to the original paper packaging.Specimen should be tested immediately after collection. If immediate testing of specimen is not possible, insert the swab into an unused general-purpose plastic tube. Ensure the breakpoint swab is level with the tube opening. Bend the swab shaft at a 180 degrees angle to break it off at the breaking point. You may need to gently rotate the swab shaft to complete the breakage. Ensure the swab fits within the plastic tube and secure a tight seal. The specimen should be disposed and recollected for retesting if untested for longer than 1 hour.
TEST PROCEDURE
Allow the test device, test sample and buffer to equilibrate to room temperature (15-30°C) prior to testing.
- Just prior to testing remove the test device from the sealed pouch just prior to the testing and lay flat on work bench.
- Push the nozzle which contains the filter onto the extraction tube. Ensure the nozzle has a tight fit.
- Hold the extraction tube vertically and add 4 drops (approximately 100 μL) of test sample solution tube into the sample well.
- Start the timer
- Read the results at 15 minutes. Do not interpret the result after 20 minutes.
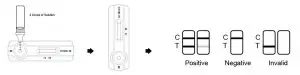
INTERPRETATION OF RESULTS
- POSITIVE:The presence of two lines as control line (C) and test line (T) within the result window indicates a positive result.
- NEGATIVE:The presence of only control line (C) within the result window indicates a negative result.
- INVALID:If the control line (C) is not visible within the result window after performing the test, the result is considered invalid. Some causes of invalid results are because of not following the directions correctly or the test may have deteriorated beyond the expiration date. It is recommended that the specimen be re-tested using a new test.
NOTE:
- The intensity of color in the test line region (T) may vary depending on the concentration of analyses present in the specimen.Therefore, any shade of color in the test line region (T) should be considered positive. This is a qualitative test only and cannot determine the concentration of analytes in the specimen.
- Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
QUALITY CONTROL
A procedural control is included in the test. A red line appearing in the control line region (C) is the internal procedural control. It confirms sufficient specimen volume and correct procedural technique. Control standards are not supplied with this test. However, it is recommended that positive and negative controls are sourced from a local competent authority and tested as agood laboratory practice, to confirm the test procedure and verify the test performance.
LIMITATIONS
- The etiology of respiratory infection caused by microorganisms other than SARS-CoV-2 will not be established with this test. The Coronavirus Ag Rapid Test Cassette (Swab) can detect both viable and non-viable SARS-CoV-2. The performance of the Coronavirus Ag Rapid Test Cassette (Swab) depends on antigen load and may not correlate with viral culture results performed on the same specimen.
- Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result.
- If the test result is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not at any time rule out the presence of SARS-CoV-2 antigens in specimen, as they may be present below the minimum detection level of the test or if the sample was collected or transported improperly.
- As with all diagnostic tests, a confirmed diagnosis should only be made by a physician after all clinical and laboratory findings have been evaluated.
- Positive test results do not rule out co-infections with other pathogens.
- Positive test results do not differentiate between SARS-CoV and SARS-CoV-2.
- Negative results should be treated as presumptive and confirmed with an FDA authorized molecular assay, if necessary, for clinical management, including infection control.
PERFORMANCE CHARACTERISTICS
1. Clinical Sensitivity, Specificity and Accuracy
Clinical Performance of the Healgen Coronavirus Ag Rapid Test (Swab) was evaluated by being involved in 6 non-laboratory sites within the US, where patients were enrolled and tested. Testing was performed by 11 non laboratorian Health Care Workers that were not familiar with the testing procedure. A total of 266 fresh nasopharyngeal swab samples was collected and tested, which includes 52 positive samples and 210 negative samples. The Healgen Coronavirus Ag Rapid Test (Swab) results were compared to results of Emergency Use Authorized RT-PCR assays for SARS-CoV-2 from nasopharyngeal swab specimen. Overall study results are shown in Table 1
Table 1: The Coronavirus Ag Rapid Test vs PCRClinical Performance of the Healgen Coronavirus Ag Rapid Test (Swab) was evaluated by being involved in 7 non-laboratory sites within the US, where patients were enrolled and tested. Testing was performed by 24 non laboratorian Health Care Workers that were not familiar with the testing procedure. A total of 317 fresh nasopharyngeal swab samples was collected and tested, which includes 61 positive samples and 256 negative samples. The Healgen Coronavirus Ag Rapid Test (Swab) results were compared to results of Emergency Use Authorized RT-PCR assays for SARS-CoV-2 from nasopharyngeal swab specimen. Overall study results are shown below.
The Coronavirus Ag Rapid Test vs PCR
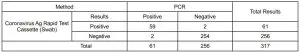
*Confidence Intervals
Relative Sensitivity: 96.72% (95%CI*: 88.65%-99.60%)Relative Specificity: 99.22% (95%CI*: 97.21%-99.91%)Accuracy: 98.74(95%CI*: 96.80%-99.66%)
2. Limit of Detection (LOD)
LOD studies determine the lowest detectable concentration of SARS-CoV-2 at which approximately 95% of all (true positive) replicates test positive. Heat inactivated SARS-CoV-2 virus, with a stock concentration of 4.6 x 105 TCID50 / mL, was spiked into negative specimen and serially diluted. Each dilution was ran in triplicate on the Healgen Coronavirus Ag test. The Limit of Detection of the Coronavirus Ag Rapid Test Cassette (Swab) is 1.15 x 102 TCID50 / mL (Table 2).
Table 2: Limit of Detection (LOD) Study Results

3. High Dose Hook EffectNo high dose hook effect was observed when testing up to a concentration of 4.6 x 105 TCID50 / mL of heat inactivated SARSCoV- 2 virus.
4. Cross ReactivityCross reactivity with the following organisms has been studied. Samples positive for the following organisms were found negative when tested with the Coronavirus Ag Rapid Test Cassette (Swab).
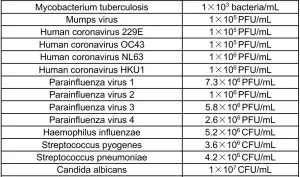


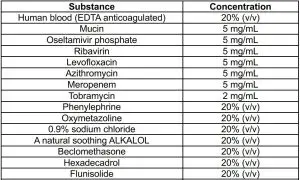
5. Interfering SubstanceThe following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal cavity or nasopharynx, were evaluated with the Coronavirus Ag Rapid Test Cassette (Swab) at the concentrations listed below and were found not to affect test performance.

INDEX OF SYMBOLS

Revision Date: 2020-10-08
![]()
[xyz-ips snippet=”download-snippet”]