User Manual

Hisense Mycoplasma PCR Detection Kit
Research Use Only. Not for Use in Diagnostic Procedures.
Introduction
HiSense Mycoplasma PCR Detection Kit utilizes the PCR, which is established as the method of choice for highest sensitivity in the detection of mycoplasma, ureaplasma and acholeplasma contamination in cell culture and other cell culture derived biologicals. The primers are specific to the highly conserved 16S ribosomal RNA sequences in the mycoplasma genome. This allows for detection of all mycoplasma species including Acholeplasma laidlawii, M. arginini, M. fermentans, M. gallisepticum, M. genitalium, M. hominis, M hyorhinis, M. orale, M. pneumoniae, Spiroplasma citri and U. urealyticum. All mycoplasma species including 8 genus and about 209 species can be detected simultaneously. The “European Pharmacopoeia” and Guideline of KFDA recommend checking for unspecific detection of Clostridium, Lactobacilllus, and Streptococcus. The 16S ribosomal RNA of other bacteria such as E.coli, Clostridium, Lactobacillus, Sterptococcus, and plant and animal cells are not amplified.
Kit Specificity
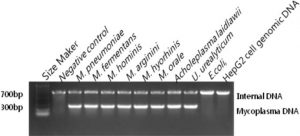
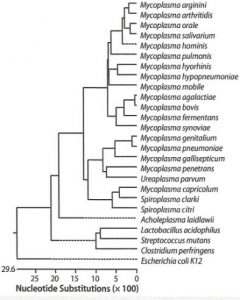
The HiSense Mycoplasma PCR Detection Kit detects Mycoplasma species simply, reliably, and rapidly. To detect the presence of these microorganisms, the assay uses the polymerase chain reaction (PCR) to amplify a target unique to a wide variety of mycoplasmas. The kit can detect more than 209 different Mycoplasma species, including Acholeplasma laidlawii and Spiroplasma citri. The kit does not detect other genera or cell-line DNA.
Kit Sensitivity
The sensitivity of the PCR using this kit is less than 10 fg of the target DNA per reaction. Sensitivity of the assay in real culture samples depends on the quality of the sample preparation.
Preventing Template Cross-Contamination
Take precautions to minimize the potential for carryover of nucleic acids from one experiment to the next. Use separate work areas and pipettes for template preparation and PCR setup steps. Use the PCR grade water delivered with the kit, aerosol-preventive filter tips and gloves.The 2× PCR master mix contains dUTP instead of dTTP. When dUTP replaces dTTP in PCR amplification, UNG treatment (Uracil-N-glycosylase, not provided in this kit) can prevent the subsequent reamplification of dU-containing PCR products. UNG acts on single- and double-stranded dU-containing DNA by hydrolysis of uracil-glycosidic bonds at dU-containing DNA sites. When this strategy is put to use, carryover contamination will be eliminated while template DNA (DNA containing T) will be left intact.
Materials Provided
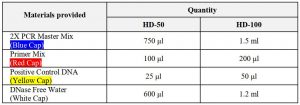
Storage ConditionUpon receipt, store at -20℃.Note:Repeat thawing reduces quality of product.If frequent freeze and thaw is needed, aliquot the products and use in order.
Test ProtocolPrepare the Template (Sample)Samples should be derived from cultures which are at 90-100% confluence. Penicillin and streptomycin in the culture media do not inhibit PCR or affect test sensitivity. To avoid false positive results, we recommend the use of the PCR grade water delivered with the kit, aerosol-preventive filter tips and gloves.
The preparation of sample screening:Sample preparation from cell culture media
- 1.2 ml liquid supernatant of the sample is transferred into a 1.5 ml tube and centrifuged (5 minutes, 1,500 rpm) to sediment cell debris.
- 1 ml of the supernatant is transferred into a 1.5 ml tube.
- Centrifuged (20 minutes, 13,000 rpm) to sediment mycoplasma particles.
- Discard supernatant and wash the pellet once with 1 ml of PBS. Repeat step 3).
- Discard supernatant and add 50 μl DNase free water or TE buffer to the pellet.
- Heat the samples at 98℃ for 10 min, and vortex for 5~10 sec. Then, centrifuge for 5 min at 12,000 rpm with a micro centrifuge. (Caution!! Be careful when you heat the sample at 98℃. Heating it in PCR machine with heating cover is recommended.)
- Transfer the heated supernatant to a fresh tube. This supernatant will be used as the template in the PCR. Take 5μl supernatant as template for qPCR reaction.
*If the template contains PCR inhibition materials, the DNA can be purified with a commercial extraction kit.
The preparation of sample for EP 2.6.7 guideline:
Genomic DNA extraction*DNA was isolated using a commercial kit, DNeasy® Blood & Tissue Kit (Cat# 69504, Qiagen, Valencia, CA) following the procedure provided by the vendor
PreparationAll centrifugation steps are carried out at room temperature (15~25°C) in a microcentrifuge.PBS is required for use in step 1. Buffer ATL is not required in this protocol.
Things to do before startingBuffer AL may form a precipitate upon storage. If necessary, warm to 56°C until the precipitate has fully dissolved. Buffer AW1 and Buffer AW2 are supplied as concentrates. Before using for the first time, add the appropriate amount of ethanol (96~100%) as indicated on the bottle to obtain a working solution. Preheat a thermomixer, shaking water bath, or rocking platform to 56°C for use in step 2.
Procedures
- Collect 1 ml cell culture (1×105 ~1×106 cells/ml) to a tube. Centrifuge for 10 min at 15,000 rpm. When using a frozen cell pellet, allow cells to thaw before adding PBS until the pellet can be dislodged by gently flicking the tube.
- Decant the supernatant and resuspend the pellet in 200 μl PBS.
- Add 20 μl proteinase K. Continue with step 4.
- Add 200 μl Buffer AL (without added ethanol). Mix thoroughly by vortexing, and incubate at 56°C for 10 min.It is essential that the sample and Buffer AL are mixed immediately and thoroughly by vortexing or pipetting to yield a homogeneous solution.
- Add 200 μl ethanol (96~100%) to the sample, and mix thoroughly by vortexing.It is important that the sample and the ethanol are mixed thoroughly to yield a homogeneous solution.
- Pipet the mixture from step 3 into the DNeasy Mini spin column placed in a 2 ml collection tube. Centrifuge at 10,000 rpm for 1 min. Discard flow-through and collection tube.
- Place the DNeasy Mini spin column in a new 2 ml collection tube, add 500 μl Buffer AW1, and centrifuge for 1 min 10,000 rpm. Discard flow-through and collection tube.
- Place the DNeasy Mini spin column in a new 2 ml collection tube, add 500μl Buffer AW2, and centrifuge for 3 min at 20,000 x g (14,000 rpm).
- Remove the 2 ml collection tube solution and centrifuge at 14,000 rpm for 3 minutes to dry the column membrane.It is important to dry the membrane of the DNeasy Mini spin column, since residual ethanol may interfere with subsequent reactions. This centrifugation step ensures that no residual ethanol will be carried over during the following elution. Following the centrifugation step, remove the DNeasy Mini spin column carefully so that the column does not come into contact with the flow-through, since this will result in carryover of ethanol. If carryover of ethanol occurs, empty the collection tube, then reuse it in another centrifugation for 1 min at 20,000 x g (14,000 rpm). 10) Place the DNeasy Mini spin column in a clean 1.5 ml microcentrifuge tube, and pipet 50 μl Buffer AE directly onto the DNeasy membrane. Incubate at room temperature for 1 min, and then centrifuge for 2 min at 10,000 rpm to elute.
Prepare for PCR
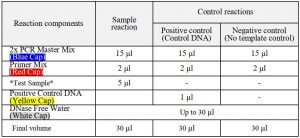
- Prepare the set of reactions listed in the following table. These include two types of control reactions: 1) positive control reaction(s) containing mycoplasma positive control template DNA, and 2) a negative control (no template). (Caution!! Don’t vigorous vortexing)
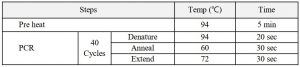
2. Set up the PCR instrument to run the PCR cycling (amplification) program specified below.
 3. Apply 10~20 μl each of PCR products to the gel electrophoresis.
3. Apply 10~20 μl each of PCR products to the gel electrophoresis.
Results
- When mycoplasma contamination exists, a band with around 250-270 bp appears. An internal DNA band with around 700 bp means the right performance of PCR reaction.

Fig. 1. Lane 1, 100 bp DNA ladder; lane 2, 10 pg M. pneumoniae DNA; lane 3, 1 pg M. pneumoniae DNA; lane 4, 100 fg M. pneumoniae DNA; lane 5, 10 fg M. pneumoniae DNA; lane 6, 1 fg M. pneumoniae DNA lane 7, 8, PCR reagent control (negative control)
Note:
- Recommend to perform one negative control without sample and one positive control reaction by adding 1μl of mycoplasma control DNA.
- If the PCR reaction is inhibited by high FBS concentration, the use of genomic DNA as a template may be helpful.
- PCR inhibiting substances may accumulate in the medium of hybridoma cell. In this case, the use of diluted sample or genomic DNA as a template may be helpful.
Hisense Mycoplasma PCR Detection Kit User Manual – Hisense Mycoplasma PCR Detection Kit User Manual –
Questions about your Manual? Post in the comments!
[xyz-ips snippet=”download-snippet”]