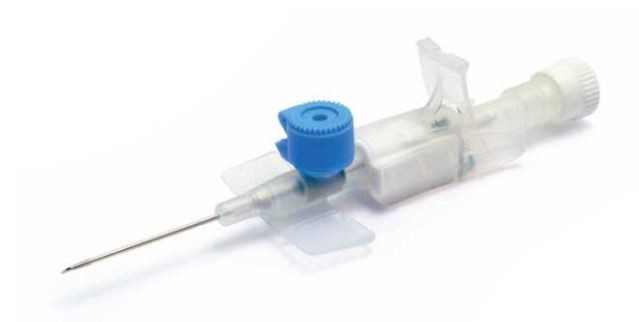
JELCO IntuitIV Safety IV Catheter
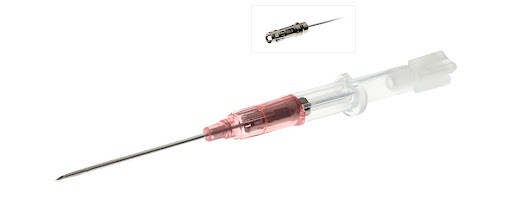
These instructions apply to the use of the following Jelco® products:
7114 to 7122 Jelco IntuitIV Safety IV Catheter™ FEP 14G to 22G7214 to 7222 Jelco IntuitIV Safety IV Catheter™ PUR 14G to 22G
These instructions contain important information for safe use of the product. Read the entire contents of these Instructions For Use, including Warnings and Cautions, before using this product. Failure to properly follow warnings, cautions and instructions could result in death or serious injury to the patient and/or clinician.
DESCRIPTION:
Each Jelco Intuit IV Safety IV Catheter™ consists of an introducer needle with an integral tip-protector. Key parts are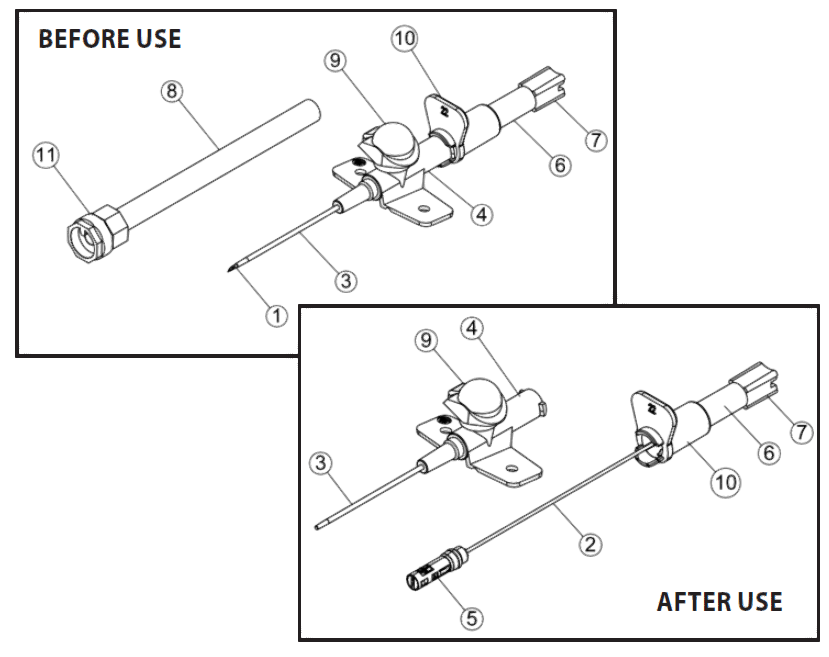
- needle bevel,
- needle,
- catheter,
- catheter hub,
- tip-protector,
- flash chamber,
- flash plug assembly,
- sheath,
- snap cap,
- thumb tab, and
- Luer lock cap.
| Gauge | 14G | 16G | 18G | 20G | 22G |
| Colour | Orange | Grey | Green | Pink | Blue |
This device is intended for single use only and is provided sterile and non-pyrogenic. The materials used to manufacture this radiopaque IV catheter do not contain latex and are PVC and DEHP free.
INDICATIONS:
- A properly placed Jelco IntuitIV Safety IV Catheter™ provides access toa vein or artery to sample blood, monitor blood pressure, or administer fluids.
- The tip-protector that locks over the needle as the needle is withdrawn helps reduce the risk of accidental needlesticks.
- These catheters may be used for any patient population with consideration given to patient size, appropriateness for the solution being infused and duration of the therapy.
WARNINGS:
- For proper use, CLINICIANS MUST BE TRAINED IN THE PRACTICE OFVENIPUNCTURE and follow the Instructions For Use. Failure to follow these instructions could result in death or serious injury to the patient and/or clinician.
- To avoid the potential of an embolus:• DO NOT CUT THE CATHETER or use sharp instruments near the catheter.• Never reinsert the introducer needle inside the catheter as it maysever it.
- To avoid an accidental needlestick:• DO NOT BEND THE NEEDLE during insertion, threading, or removalof the catheter assembly.• If venipuncture is not successful, engage the safety mechanism and discard both the needle and the catheter.• In the unlikely event that the device does not lock out; dispose of the sharp immediately into an approved sharps container.
- Needle is not intended to be used with a guidewire – use will prevent activation of safety tip-protector
- Do not manipulate the tip-protector assembly before or after use.
- Do not use under high pressure conditions. To avoid leakage, maximum pressure for side-port catheters is 45 psi.
CAUTIONS: Product is sterile, non-toxic and non-pyrogenic unless package is open, wet, or damaged. Discard if open, wet, or damaged.
PRECAUTIONS:
- This device is designed to help reduce the risk of accidental needlesticks.In addition to these instructions for use, it is recommended that the health care professional follow the recommendations set forth by the CDC and OSHA standards (USA) or local equivalent for bloodborne pathogens when starting, maintaining, or discarding any IV catheter to avoid the risk of exposure to blood.
- follow current institutional policies and procedures for catheter insertion, maintenance and removal.
- The catheter is shorter than the introducer needle. Therefore, blood flashback may occur before the catheter tip is fully in the blood vessel. If necessary, slightly advance the catheter and needle together to assure full catheter entry into the blood vessel lumen. To avoid inadvertently puncturing the posterior wall of the vessel, lower the needle until it is parallel to the skin.
- Ensure fluid administration/hub connection is secure in order to prevent leaking.
- Ensure stabilization of catheter to the patient. Improper stabilization may lead to loss of vascular access.
INSTRUCTIONS FOR USE:
Due to the risk of bloodborne pathogen exposure, follow StandardPrecautions during placement, use and removal of an IV catheter.
- Select and prepare site per institutional policy. Apply tourniquet.
- Remove sheath in straight outward motion and inspect device. Ensure snap cap is closed and the catheter hub is fully seated and verify the needle bevel is in the up position.
- find a stable position to hold the Jelco Intuit IV Safety IV Catheter™ by holding the wings with index and middle finger and place the thumb at the flash plug assembly.
- Anchor the blood vessel with gentle skin traction and insert the needle into the skin and blood vessel at an appropriate angle.
- Blood flashback into the flash chamber confirms blood vessel entry.
- Decrease angle and insert device slightly to assure catheter entry into the blood vessel.
- Remove tourniquet.
- Before removing needle, apply digital pressure to the blood vessel distal to the catheter tip and secure the catheter hub, remove the needle by pulling straight back. The tip-protector will automatically engage over the needle tip.Note: A slight resistance will occur as the protected needle is removed from the catheter hub.
- Note: Do not reinsert the tip-protector into the hub after it has been removed.
- Connect IV administration line and dress site per institutional policy.
- Immediately discard unit into a puncture resistant, leak-proof, disposable sharps container.
Jelco IntuitIV Safety IV Catheter, Jelco design mark and Smiths Medical design marks are trademarks of Smiths Medical. The symbol ® indicates that it is registered in the U.S. Patent and Trademark Office and certain other countries. All other names and marks mentioned are the trade names, trademarks or service marks of their respective owners. There is no connection between Smiths Medical and Intuitive Surgical. ©2012 Smiths Medical. All rights reserved.
- Single use.
- Do Not Reuse: Medical devices require specific material characteristics to perform as intended.
- These characteristics have been verified for single use only.
- Any attempt to re-process the device for subsequent re-use may adversely affect the integrity of the device or lead to deterioration in performance.
- Sterile unless unit container is opened or damaged.
- Destroy after single use.
- Do not resterilize.

References
Smiths Medical US Homepage: Portex Medex Deltec Level1 BCI CADD Pneupac Surgivet Graseby Jelco Medfusion
Smiths Medical US Homepage: Portex Medex Deltec Level1 BCI CADD Pneupac Surgivet Graseby Jelco Medfusion
Smiths Medical US Homepage: Portex Medex Deltec Level1 BCI CADD Pneupac Surgivet Graseby Jelco Medfusion
[xyz-ips snippet=”download-snippet”]