COVID-19 Antigen Rapid Test (Latex)Instructions For Use
【PRODUCT NAME】
COVID-19 Antigen Rapid Test (Latex)
【SUMMARY】
The novel coronaviruses belong to the β genus. COVID-19 is anacute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novelcoronavirus are the main source of infection; asymptomaticinfected people can also be an infectious source. Based on thecurrent epidemiological investigation, the incubation period is 1 to14 days, mostly 3 to 7 days. The main manifestations include fever,fatigue and dry cough. Nasal congestion, runny nose, sore throat,myalgia and diarrhea are found in a few cases.
【PACKING SPECIFICATIONS】
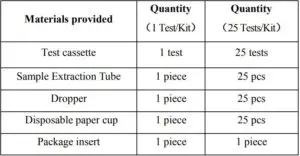
1 Test/Kit, 25 Tests/Kit.
【INTENDED USE】
The COVID-19 Antigen Rapid Test (Latex) is suitable for the qualitative detection of novel coronavirus in posterior oropharyngeal saliva, sputum and stool samples. It provides an aid in the diagnosis of infection with novel coronavirus.The COVID-19 Antigen Rapid Test (Latex) is to be used in conjunction with clinical manifestations and other laboratory test results to assist in the diagnosis of patients with suspected SARSCoV-2 infection. The test is only to be used by medical professionals. It provides only an initial screening test result and more specific alternative diagnosis methods should be performed in order to obtain the confirmation of SARS-CoV-2 infection.
【PRINCIPLE】
The novel coronavirus invades human cells by the specific binding of its spike glycoprotein (ligand) to the ACE2 receptor located on human cellular membrane. In this test the ACE2 receptor has been substituted for antibody to establish a novel ligand-receptor chromatography test kit for rapid detection of the novel coronavirus. In clinical practice, the test can be used for rapid detection of SARS-Cov-2 and all its mutants in posterior oropharyngeal saliva, sputum and stool samples from individuals. The test only takes 15 minutes to perform and is much easier and faster than nucleic acid testing (RT-PCR). It has been found that SARS-CoV-2 virus has evolved into more contagious mutants through mutations in S1 proteins (such as D614G) that have stronger binding to ACE2 receptors. Given the current assay format, based on ACE-2 receptor binding, the test should be able to also detect such mutants.The test kit contains a nitrocellulose (NC) membrane on towhich, the rabbit anti-S1 protein of novel coronavirus antibodies iscoated at the T line region, the goat anti-rabbit IgG polyclonalantibody is coated at the control line region (C). Latex-labeledACE2 protein and Latex-labeled rabbit IgG are embedded in thereagent pad.To perform the test three drops of the sample are added to thesample well and the sample flows from the bottom to the top bycapillary effect. After a 15 min incubation if the patient samplecontains the virus, the latex labeled ACE2 protein will be bound bythe S1 protein of virus, and then captured by anti-S1 protein antibodies coated on the T line region. If the sample does not contain the virus, the latex-labeled ACE2 protein will not be captured by anti-S1 protein antibodies coated on the T line region, therefore, no T line will appear. Whether the sample contain thevirus or not, the latex-labeled rabbit IgG will react with the goatanti-rabbit IgG polyclonal antibody coated onto the control lineregion (C) and a colored line will appear in the control area.Once the testing is finished, the amount of latex-ACE2 proteinbound on the T line is directly proportional to the concentration ofnovel coronavirus in the sample, while the amount of latex boundon the control line C is not related to the to the concentration ofcoronavirus in the sample.
【KIT COMPONENTS】

【STORAGE AND STABILITY】
he test is valid for 12 months if all components are kept packaged in the sealed pouch, protected from light and stored correctly at 2℃~30℃. After opening the reagent package, the test should be performed within one hour.
Please refer to the packing of the product for the manufacture date and expiration date.
【SPECIMEN COLLECTION AND PREPARATION】
- .The COVID-19 Antigen Rapid Test (Latex) can be applied toposterior oropharyngeal saliva, sputum and stool.
- Posterior oropharyngeal saliva: Perform hand hygiene with soap and water /alcohol-based hand rub. Open the container. Make a “Kruuua” noise from the throat to clear the saliva from deep throat,then spit saliva (about 2 ml) into the container. Avoid any saliva contamination of the outer surface of the container. Optimal timing of specimen collection:After getting up and before brushing teeth, eating or drinking.
- The test should be performed immediately after the sample iscollected. Do not leave the sample at room temperature for more than 2 hours. Specimens may be stored at -20°C for up to 1 month prior to testing.
- If specimens are to be transported, they should be packed incompliance with local regulations covering the transportation of etiological agents.
- If samples are stored at -20℃ they must be returned to roomtemperature, thawed completely and fully mixed prior to testing. The samples can be frozen and thawed once and repeated freezing and thawing should be avoided.
【TESTING PROCEDURE】
Please read the instructions carefully and allow the test deviceand specimens to equilibrate to temperature (15℃-30℃) priorto testing.
- Posterior oropharyngeal saliva, sputum sample: Unscrew theSample Extraction Tube and transfer approximately 200μL of fresh saliva or sputum from container into the Sample Extraction Tube and shake and mix completely.

- .Stool Sample: Unscrew the Sample Extraction Tube and use the sampling rod to pick up approximately 30mg of fresh stool samples (equivalent to the size of a match head). Place the sampling rod into the Sample Extraction Tube and shake and mix completely until all the stool is dissolved.

- Take the test cassette from the packaging bag, place it on a table, cut off the protrusion of the collection tube, and add 3 drops of the sample into the sample hole vertically.
- Read the result after 15 minutes. If left unread for 20 minutes or more the results are invalid, and a repeat test is recommended.
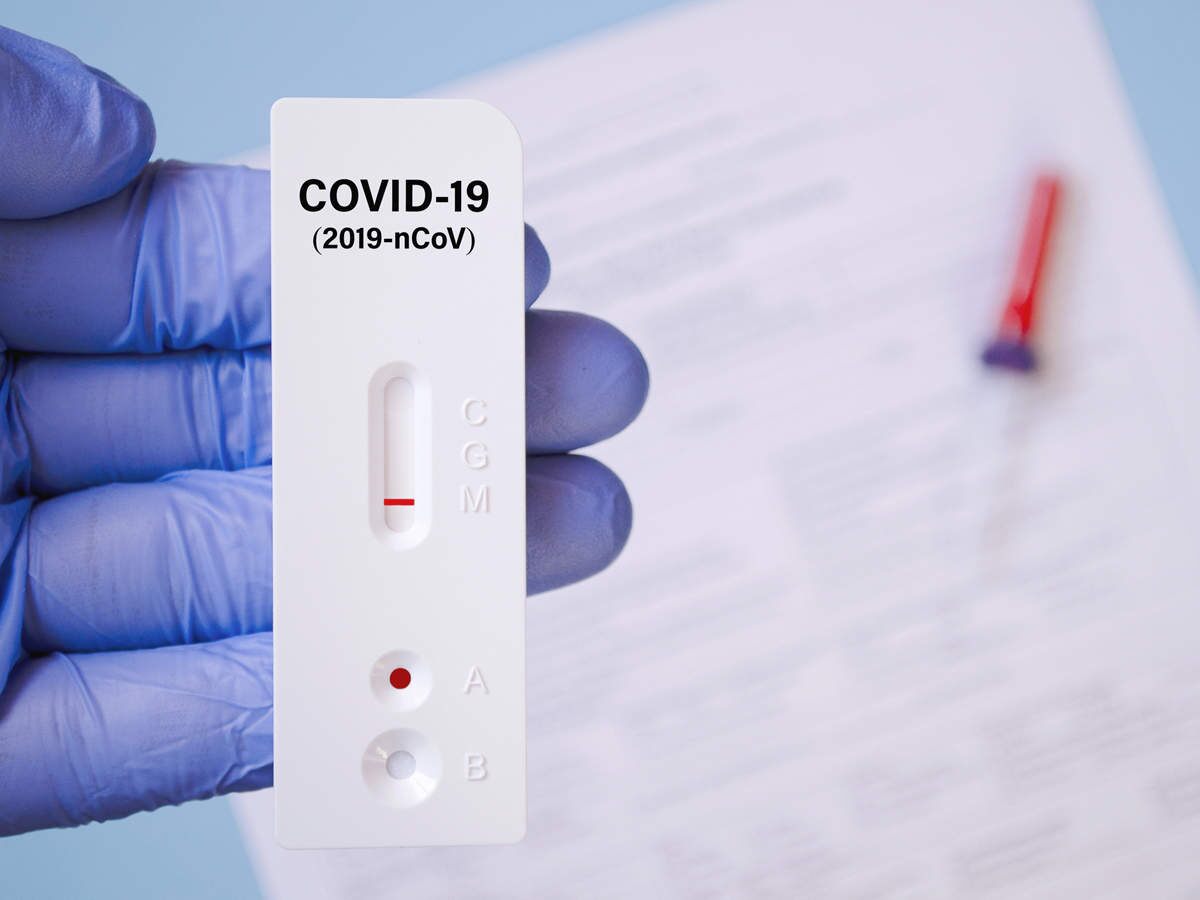
【INTERPRETATION OF RESULTS】
Positive (+): Two colored lines appear
One colored line should always appear in the control line region (C) and another line should be in the T line region.*NOTE: The intensity of the color in the test line regions mayvary depending on the concentration of SARS-CoV-2 present inthe specimen. Therefore, any shade of color in the test line regionshould be considered positive and recorded as such.
Negative (-): One colored line appears in the control line region (C)
No line appears in the T line region.
Invalid: Control line fails to appear.
Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.

【QUALITY CONTROL PROCEDURES】
Internal procedural controls are included in the test to confirm if enough specimen volume is added and correct procedural technique followed. A colored line appearing in the control region (C) is an indication that the test results are valid. Control standards are not supplied with this kit, however, it is recommended that positive and negative controls be tested as good laboratory practice test procedure and to verify the test performance.
【LIMITATIONS】
- COVID-19 Antigen Rapid Test (Latex) is only applicable to posterior oropharyngeal saliva, sputum and stool samples. Use of Blood, serum, plasma and other samples such as nasal swabs have not been verified. If the sputum sample is negative and the clinical indications suggest a Covid-19 infection, testing of a stool sample is recommended. If any sample tests positive, please go to the hospital for further clinical diagnosis. Neither the quantitative value nor the rate of increase in the concentration of SARS-CoV-2 can be determined by this qualitative test.
- COVID-19 Antigen Rapid Test (Latex) will only indicate thepresence to SARS-CoV-2 in the specimen and should not be used as the sole criteria for the diagnosis of SARS-CoV-2 infections.
- As with all diagnostic tests, all results must be considered with other clinical information available to the physician.
- If the test result is negative and clinical symptoms persist,additional follow-up testing using other clinical methods issuggested. A negative result at any time does not preclude the possibility of SARS-CoV-2 infection.
- The potential impacts of vaccines, antiviral therapeutics,antibiotics, chemotherapeutic or immunosuppressant drugs have not been evaluated in the test.
- Due to inherent differences between methodologies, it is highlyrecommended that, prior to switching from one technology to the next, method correlation studies are undertaken to qualifytechnology differences. One hundred percent agreement between the results should not be expected due to differences between technologies.
- Performance has only been established with the specimen types listed in the Intended Use. Other specimen types have not beenevaluated and should not be used with this assay.
- False results may occur if specimens are tested past 2 hours ofcollection. Specimens should be test as quickly as possible after specimen collection.
- Negative results, from patients with symptom onset beyond five days, should be treated as presumptive and a confirmation with a molecular assay, if necessary, may be performed.
- A false-negative test result may occur if the level of viralantigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly; therefore, a negative test result does not rule out the possibility of SARS CoV2 infection.
- This impact of testing patient samples which have been storedin Viral transport medium (VTM) has not been validated and assuch the results may be compromised.
- Negative results do not rule out SARS-CoV-2 infection,particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals
【PERFORMANCE CHARACTERISTICS】
1. Limit of Detection
The limit of detection (LOD) of COVID-19 Antigen RapidTest (Latex) is 0.5ng/mL SARS-COV-2 spike glycoprotein.
2. Sensitivity and Specificity
The COVID-19 Antigen Rapid Test (Latex) was comparedwith a leading commercial reagent (PCR); the results show that COVID-19 Antigen Rapid Test (Latex) has high sensitivity andspecificity.
Posterior oropharyngeal Saliva sample:

Relative Sensitivity: 90.00%(95%CI:79.49%~96.24%)Relative Specificity: 100.00%(95%CI:88.43%~100.00%)Accuracy: 93.33%(95%CI:86.05%~97.51%)
Sputum sample:

Relative Sensitivity: 95.00%(95%CI:86.08%~98.96%)Relative Specificity: 100.00%(95%CI:88.43%~100.00%)Accuracy: 96.67%(95%CI:90.57%~99.31%)
Stool sample:
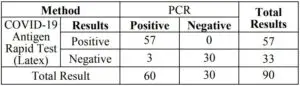
Relative Sensitivity: 95.00%(95%CI:86.08%~98.96%)Relative Specificity: 100.00%(95%CI:88.43%~100.00%)Accuracy: 96.67%(95%CI:90.57%~99.31%)
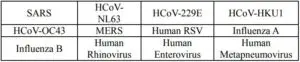
3. Cross-reactivity:
The following cross-reactive substances have been tested using COVID-19 Antigen Rapid Test (Latex) and no cross-reactivity was observed.
4. Interfering Substances:
The following compounds have been tested using the COVID-19 Antigen Rapid Test (Latex) and no interference was observed.

 【WARNINGS AND PRECAUTIONS】
【WARNINGS AND PRECAUTIONS】
- For in vitro diagnostic use only. The test is intended for professional use only and is limited to medical institutions.
- The storage and operation of the kit should comply with the requirements in the manual, otherwise there will be potential for influencing the test results.
- Do not freeze reagents.
- Reagent to avoid contamination.
- There is animal-derived protein material in the kit, so the used product should be treated as bio-waste.
- Materials in the testing process may be infectious. These should be treated according to laboratory biosafety requirements based on biohazardous substances.
- Do not use the Test cassette if the pouch is damaged or the seal broken.
- If part of the test paper in the strip is out of the test window, or more than 2 mm of filter paper or latex pad is exposed in the test window, do not use as the test result will be s invalid.
【REFERENCE】
- Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res 2011;81:85-164. PMID:22094080 DOI:10.1016/B978 0- 12-385885-6.00009-2.
- Su S, Wong G, Shi W, et al. Epidemiology, genetic recom bination, and pathogenesis of coronaviruses. Trends Microbiol 2016;24:490-502. PMID:27012512 DOI:10.1016/j.tim.2016.03.0 03.
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019;17:181 192.PMID:30531 947 DOI:10.1038/s41579-018-0118-9.
【EFFECTIVE DATE AND VERSION】
Effective Date:2020-10-09Version:1.0
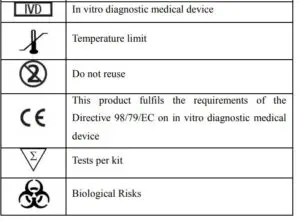
![]() Note:Please refer to the table below to identify various symbols.
Note:Please refer to the table below to identify various symbols.

Joinstar Biomedical Technology Co.,Ltd.Address:10th Floor ,Administration Building,NO.519,XingGuo RD.,Yuhang Economic andTechnological Development Zone, Hangzhou ,Zhejiang, China, 311188E-mail: [email protected]Tel: 0086-571-89023160Fax: 0086-571-89028135 Lotus NL B.V.Address: Koningin Julianaplein 10, 1e Verd, 2595AA,The Hague, Netherlands.E-mail:[email protected]Tel:+31644168999
JoinStar COVID-19 Antigen Rapid Test (Latex) Instructions for Use – JoinStar COVID-19 Antigen Rapid Test (Latex) Instructions for Use –
[xyz-ips snippet=”download-snippet”]