[Product name]SARS-CoV-2-Antigen-Rapid Test Strip(Colloidal gold immunochromatography)[Model]50 Teststrips/Kit
[Usage]
The COVID-19 rapid test kit is an immunochromatographic test system for a rapid, qualitative detection of the antigen of the severe acute respiratory syndrome SARS-CoV-2 (COVID-19) antigen in human specimens and can be used for a diagnosis of corona infectious disease (COVID-19) in vitro, which is caused byCOVID-19.The COVID-19 rapid test kit offers preliminary test results; if the results are negative, a COVID-19 infection cannot be ruled out. The test cannot be used as the sole basis for treatment or other guiding decisions. Only suitable for in vitro diagnosis.
[Summary]
Novel coronavirus pneumonia (Coronavirus disease 19, COVID-19) is an infectious disease caused by COVID-19 infection. Most patients have lung disease, a few patients have digestive system and nervous system damage, and antigen can be detected in human respiratory secretions about 2 days after infection. The detection of antigen is helpful for the diagnosis of COVID-19 infection.
[Principle]
The COVID-19 Antigen Lateral Flow Assay kit is based on colloidal gold immunochromatography to detect COVID-19 S protein in respiratory secretions other specimens. When the specimen is added into the test device, the specimen is absorbed into the device by capillary action, mixes with the gold-labelled antibody, and flows across the pre-coated membrane.The COVID-19 antigen in specimen captured by the gold-labelled antibody S1a bound to antibody S1 immobilized in the Test Region(T) of the membrane, and this produces a coloured test band thatindicates a positive result.When there is no COVID-19 antigen in the specimen or the concentration is lower than the detection limit of the test, there is not a visible coloured band in the Test Region (T) of the device.
This indicates a negative result.To serve as a procedure control, a coloured line will appear at the Control Region (C), if the test has been performed properly.
[Precaution]
- FOR USE BY MEDICAL PROFRESSIONAL ONLY.
- Proper specimen collection storage and transit are critical to the performance of this test.
- Use only once.
- Do not touch the reaction area of test strip.
- Do not use test kit beyond the expiration date.
- Do not use the kit if the pouch is punctured or sealed not well.
- Testing should be applied by professionally trained staff working in certified laboratories or clinics.
- The test result should be interpreted by the physician along with clinical findings and other laboratory test results.
- Dispose of test cards and items in contact with samples as medical waste after use.
- Do not freeze.
[Material]
Components of the Test Kit
- 50 single packed Test Strips
- 2 x 15 ml solution buffer (50 tests), 10 mM PBS (pH 7,4).
- Instruction for Use.
- 50 single packed Flock Swabs.
- 50 Virus Preservation Tube.
Material Required but Not Provided with the Kit
- Timer.
- Personal Protective Equipment, such as protective gloves, medical mask, goggles and lab coat.
- Appropriate biohazard waste container.
- Desinfectants.
[Lagerung und Stabilität]
- Stored at 2°C~30°C in the sealed pouch up tot he expiration date printed on the package.
- When the humidity of the test environment is more than 60%, the test strip needs to be used immediately after the opening of the aluminium foil bag. When the humidity ofthe test environment is less than 60%, the test strip needs to be used within 1 hour after the opening of the aluminium foil bag.
[Specimen Collection and Preparation]
The appropriate sample type for the antigen test is respiratory secretion (nasopharynx swab).
Swab Samples
- Nasopharyngeal swab: collect one nasopharyngeal specimen per test using the swab provided. Or a suitable polyester swab with an elastic handle and aseptic cotton, synthetic fibre or foam.
- The sample must be eluted within one hour of collection andtested as soon as possible.
- Add 0.5 ml (10 drops) of the solution buffer into the Virus Preservation Tube.
- Insert the swab into the tube and rotate it at least 5 times in the liquid and squeeze it out several times on the wall of the tube.
- Divide the swab at the breaking point and leave the part with the swab in the tube.The sample solution in the tube is now prepared for detection.
[Test Procedure]
- Nehmen Sie einen Teststreifen aus seiner versiegelten Verpackung.
- Insert the test strip into the prepared virus preservation tube with the prepared sample solution (~ 0.5 ml), whereby the liquid surface must not exceed the max. Line.
 Leave the test strip in the tube, place it firmly in a vertical position and do not move it until you receive the test result.
Leave the test strip in the tube, place it firmly in a vertical position and do not move it until you receive the test result. - You can read the test result within 15 minutes.
- When the test is complete, keep the virus preservation tube closed without removing the test strip or swab and dispose it properly.
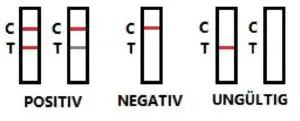
[Result Interpretation]
- The appearance of two lines (test and control), regardless of theintensity of the test line, indicates a positive result.
- A single control line shows a negative result.
- If the control line does not appear, the result is invalid and the test should be repeated.
[Quality Control]
A procedural control is included in the test. A coloured line appears in the control region (C) and is an internal procedural control. It confirms adequate sample volume, adequate membrane wicking, and correct procedural technique.Good laboratory practice recommends the use of control materials. Users should follow appropriate local and federal guidelines for the frequency of testing by external quality control materials.
[Limitations of Procedure]
- Only applicable for qualitative tests and auxiliary diagnoses.
- The concentration of the COVID-19 antigen will decrease hen the patient has developed many antibodies or has been treated.This can lead to negative test results. Confirm the infection in combination with clinical manifestations of patients or othermethods.
- Avoid the use of special samples: high-fat blood serum (triglyceride concentration> 25mg / ml), bilirubin serum (> 0.2 mg / ml), hemolytic serum (hemoglobin concentration> 5.0 mg / ml) samples may appear as red background during the test and thereby influence the appearance of the test results.
- The commonly used antiviral drugs such as Epistin-HidroChloride (<4mg / L), Ribavirin (<40 mg / L), Interferon (<200 mg / L), Oseltamivir (<30 mg / L), Abidol (40 mg / l), levofloxacin (<200 mg / l), azithromycin (<100 mg / l), ceftriaxone (<400 mg / l) and meropenem (<200 mg / l) do not affect the detection.
- Heparin, sodium citrate, EDTA and other anticoagulants will not have no effect on the detection of this test.
[Performance Characteristics]
- Clinical sensitivity, specificity and accuracy The clinical performance of the COVID-19 rapid test kit was evaluated at the hospitals (A) Beijing Puren, (B) Beijing 302, (C) Tianjin Haihe, which admitted and tested patients (no laboratories). The tests were performed by healthcare workers with laboratory experience who were familiar with the test procedure. Samples were taken within 7 days of the onset of symptoms. A total of 786 fresh nasopharyngeal swab specimens were collected and tested, including 333 positive and 453 negative specimens.The results of the COVID-19 apid test kit were compared with the results of RT-PCR tests (positive: ≤36). The overall results are shown in the following table:
 Sensitivity: 98,5% (95%CI*: 97,20-99,80%)Spezificity: 99,3% (95%CI*: 98,50-100%)Coincidence Rate: 99,0% (95%CI*: 98,30-99,70%)Kappa : 0,979 (P<0.0001)* Confidence-Interval
Sensitivity: 98,5% (95%CI*: 97,20-99,80%)Spezificity: 99,3% (95%CI*: 98,50-100%)Coincidence Rate: 99,0% (95%CI*: 98,30-99,70%)Kappa : 0,979 (P<0.0001)* Confidence-Interval - Limit of detection LOD studies determine the lowest detectable concentration of SARS-CoV-2 at which around 95% of all (truly positive) replicates test positive. Heat-inactivated SARS-CoV-2 viruses with a starting concentration of 4.6 x 10 5 TCID50 / ml (tissue culture infection dose of 50%) were found in negative samples transferred and serially diluted. Each dilution was tested in triplicate with the COVID-19 Antigen Rapid Test Kit. The detection limit of the COVID-19 rapid test kit is 1.15 x 102 TCID50 / ml.
- Prozone effect In the investigation with heat-inactivated SARS-CoV-2 viruses, no prozone effect was found up to a concentration of 4.6 x 10 5 TCID50 / ml.
- Cross reactivity This test system has no cross-reactivity with the endemic human coronavirus 229E / OC43 / NL63, influenza A virus, influenza B virus, respiratory syncytial virus, MERS virus, adenovirus, the EB virus, measles virus, cytomegaly virus, Rotavirus, Norwalk Virus, Mumps Virus, Varicella Zoster Virus, Mycoplasma Pneumoniae,Human Metapneumovirus.
Koch Biotechnology (Beijing) Co., Ltd.No. 16, Chulin Street, Daxing District, Beijing, China
Tel.: +86 (10) 6125 0336, E-mail: [email protected]www.kochbiotech.com https://vimeo.com/482996160
Wellkang Ltd. (www.CE-marking.eu)16 Castel St, Dover, CT16 1PW, UK
[Date of approval and revision of the instructions for use]:Approved November 24th 2020, version number: CE-NCV10 REV.04
Koch Biotech COVID-19 Rapid Test Strip Instructions For Use – Koch Biotech COVID-19 Rapid Test Strip Instructions For Use –
[xyz-ips snippet=”download-snippet”]


 Leave the test strip in the tube, place it firmly in a vertical position and do not move it until you receive the test result.
Leave the test strip in the tube, place it firmly in a vertical position and do not move it until you receive the test result.