 E1 Series Ear SensorsLNCS®/M-LNCS® Single Patient Use Ear Sensor
E1 Series Ear SensorsLNCS®/M-LNCS® Single Patient Use Ear Sensor
![]()
![]()
  |
  |
  |
  |
  |
  |
DIRECTIONS FOR USE
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
|
When Used with: |
Masimo |
| > 30 kg | |
| Application Site | Ear |
| Saturation Accuracy, No Motion | ± 2.5% |
| Pulse Rate Accuracy, No Motion | ± 3 bpm |
| Low Perfusion Accuracy | SpO2 ± 2.5% |
| Pulse ± 3 bpm |
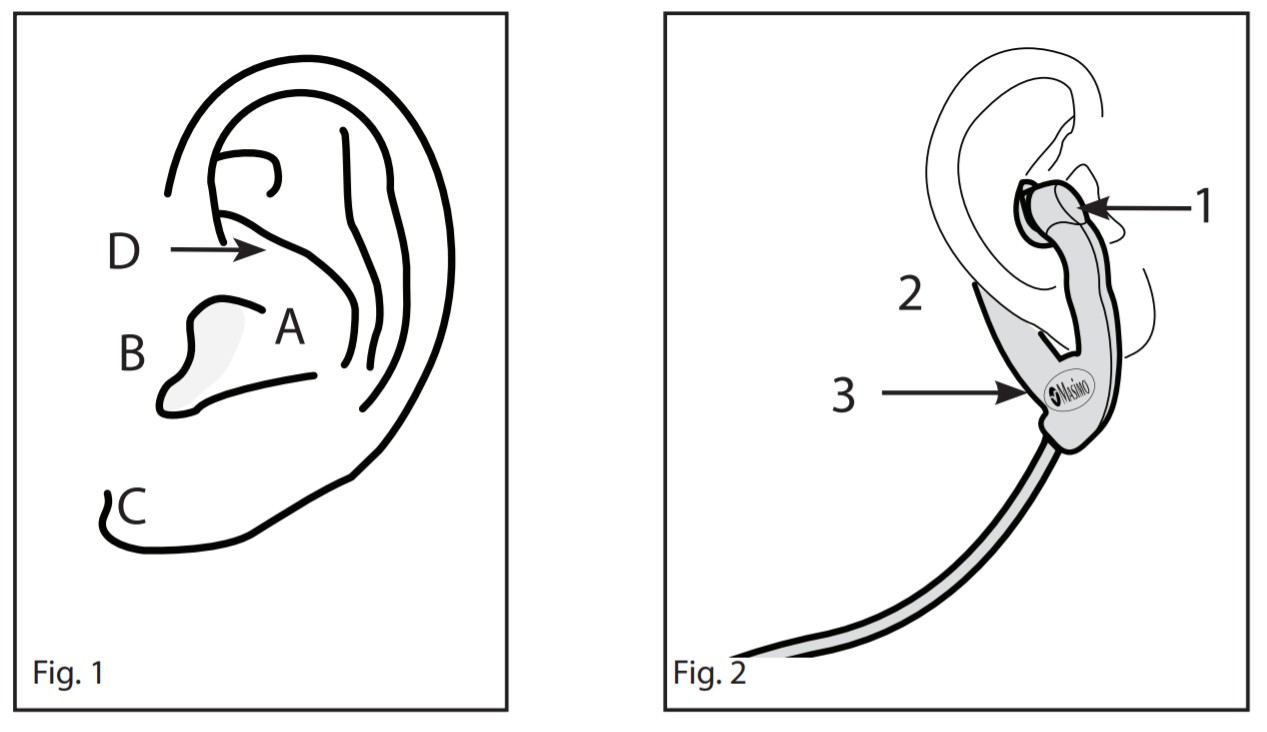
DESCRIPTIONThe E1 Series Ear Sensors are intended for use only with instruments containing Masimo SET® MS-2000 (Version 4.8 or higher) technology, Masimo rainbow® SET® MX technology.The E1 Ear Sensor has been validated on Masimo SET Oximetry technology, which is included in the Radical-7®, Rad-8®, Rad5®, Rad-87®, and Rad-57® instruments.WARNING:Masimo sensors and cables are designed for use with instruments containing Masimo SET® oximetry or licensed to use Masimo sensors.CONTRAINDICATIONSThe E1 Ear Sensor is contraindicated for patients who exhibit allergic reactions to foam rubber products and/or adhesive tape or for patients with signs of redness, swelling, infection, or skin breakdown at the sensor application site, including the inner aspect of the ear and behind the ear. The sensor cannot be placed over areas of the ear that have piercings.Refer to Fig. 1. The sensor should not be used on patients if the inner aspect of the ear (cavum conchae [A]) (reference is also made to the tragus [B], ear lobe [C], and crus of helix [D].) is not large enough to accommodate the emitter limb of the sensor without touching the tragus and/or crus of the helix. The sensor is not intended for use on a monitoring site for prolonged periods. If extended monitoring is required, the sensor must be moved to the opposite ear. If the sensor cannot be moved, the use of a Masimo adhesive sensor on a peripheral site, such as the digit, is recommended.Because individual skin conditions and perfusion levels affect the ability of the site to tolerate sensor placement, it may be necessary to move the sensor frequently.WARNINGS
- Do not use the E1 Ear Sensor sensor on any site other than the inner aspect of the ear (cavum conchae). This may result in inaccurate readings due to tissue thickness.
- All sensors and cables are designed for use with specific monitors. Verify the compatibility of the monitor, cable, and sensor before use, otherwise degraded performance and/or patient injury can result.
- The site must be checked frequently or per clinical protocol to ensure adequate adhesion, circulation, skin integrity, and correct optical alignment.
- Exercise extreme caution with poorly perfused patients; skin erosion and pressure necrosis can be caused when the sensor is not frequently moved. Assess site as frequently as every (1) hour with poorly perfused patients and move the sensor if there are signs of tissue ischemia.
- During low perfusion, the sensor site needs to be assessed frequently for signs of tissue ischemia, which can lead to pressure necrosis.
- With very low perfusion at the monitored site, the reading may read lower than core arterial oxygen saturation.
- Do not use tape to secure the sensor to the site; this can restrict blood flow and cause inaccurate readings. The use of additional tape can cause skin damage, and/or pressure necrosis or damage the sensor.
- Sensors applied too tightly or that become tight due to edema will cause inaccurate readings and can cause pressure necrosis.
- Misapplied sensors or sensors that become partially dislodged may cause incorrect measurements.
- Venous congestion may cause by the reading of actual arterial oxygen saturation. Therefore, assure proper venous outflow from the monitored site. The sensor should not be below heart level (e.g. sensor on hand of a patient in a bed with an arm dangling to the floor, Trendelenburg position).
- Venous pulsations may cause erroneous low SpO2 readings (e.g. tricuspid valve regurgitation, Trendelenburg position).
- The pulsations from intra-aortic balloon support can be additive to the pulse rate on the oximeter pulse rate display. Verify the patient’s pulse rate against the ECG heart rate.
- The sensor should be free of visible defects, discoloration and damage. If the sensor is discolored or damaged, discontinue use. Never use a damaged sensor or one with exposed electrical circuitry.
- Carefully route cable and patient cable to reduce the possibility of patient entanglement or strangulation.
- If using pulse oximetry during full body irradiation, keep the sensor out of the radiation field. If the sensor is exposed to the radiation, the reading might be inaccurate or the unit might read zero for the duration of the active radiation period.
- Do not use the sensor during MRI scanning or in an MRI environment.
- High ambient light sources such as surgical lights (especially those with a xenon light source), bilirubin lamps, fluorescent lights, infrared heating lamps, and direct sunlight can interfere with the performance of the sensor.
- To prevent interference from ambient light, ensure that the sensor is properly applied, and cover the sensor site with opaque material, if required. Failure to take this precaution in high ambient light conditions may result in inaccurate measurements.
- High levels of COHb or MetHb may occur with a seemingly normal SpO2. When elevated levels of COHb or MetHb are suspected, laboratory analysis (CO-Oximetry) of a blood sample should be performed.
- Elevated levels of Carboxyhemoglobin (COHb) may lead to inaccurate SpO2 measurements.
- Elevated levels of Methemoglobin (MetHb) will lead to inaccurate SpO2 measurements.
- Elevated Total Bilirubin levels may lead to inaccurate SpO2 measurements.
- Intravascular dyes such as indocyanine green or methylene blue or externally applied coloring (such as indelible ink) may lead to inaccurate SpO2 measurements.
- Inaccurate SpO2 readings may be caused by severe anemia, low arterial perfusion or motion artifact.
- To prevent damage, do not soak or immerse the sensor in any liquid solution. Do not attempt to sterilize the sensor.
- Do not modify or alter the sensor in any way. Alteration or modification may affect performance and/or accuracy.
- Do not attempt to reuse on multiple patients, reprocess, recondition or recycle Masimo sensors or patient cables as these processes may damage the electrical components, potentially leading to patient harm.
INSTRUCTIONSA) Site selectionCAUTION: Before using the sensor, ensure that the sensor is physically intact, with no broken or frayed wires or damaged parts.
- Refer to Fig 1. The preferred measuring site is the hollow of the inner aspect of the ear (cavum conchae [A]) (Reference is also made to the tragus [B], ear lobe [C] and crus of helix [D].)
- The site should be free of debris. Prior to sensor placement, the site should be checked to ensure that it is clean, without signs of redness, swelling, infection, or breakdown.
B) Attaching the Sensor to the Patient
- Open the pouch and remove the sensor.
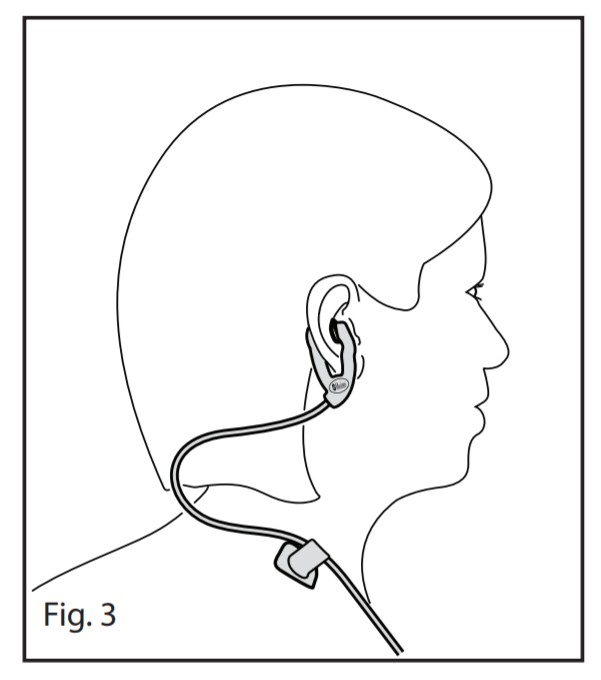
- Refer to Fig 2. Orient the sensor to ensure that the star (1) on the emitter limb is in front of the patient’s ear. Gently pull the emitter and detector limb apart to separate, so they will clear the ear lobe. Place the emitter limb inside the ear so that it rests on the hollow of the inner aspect of the ear (cavum conchae).
- Place the detector limb on the back of the ear (2). The detector limb pad should lay flat on the back of the ear, without any part of the pad folding under.
- Position the bottom of the sensor notch (3) so that it fits under the smallest portion of the ear lobe. For the patient’s comfort, the E1 Ear Sensor can be adjusted after it is placed. Ensure that the sensor is not compressing any skin. It is recommended that the emitter not touch the tragus.
- If the Masimo Ear sensor does not fit properly on the ear, consider using a Masimo reusable or adhesive sensor on another measuring site.
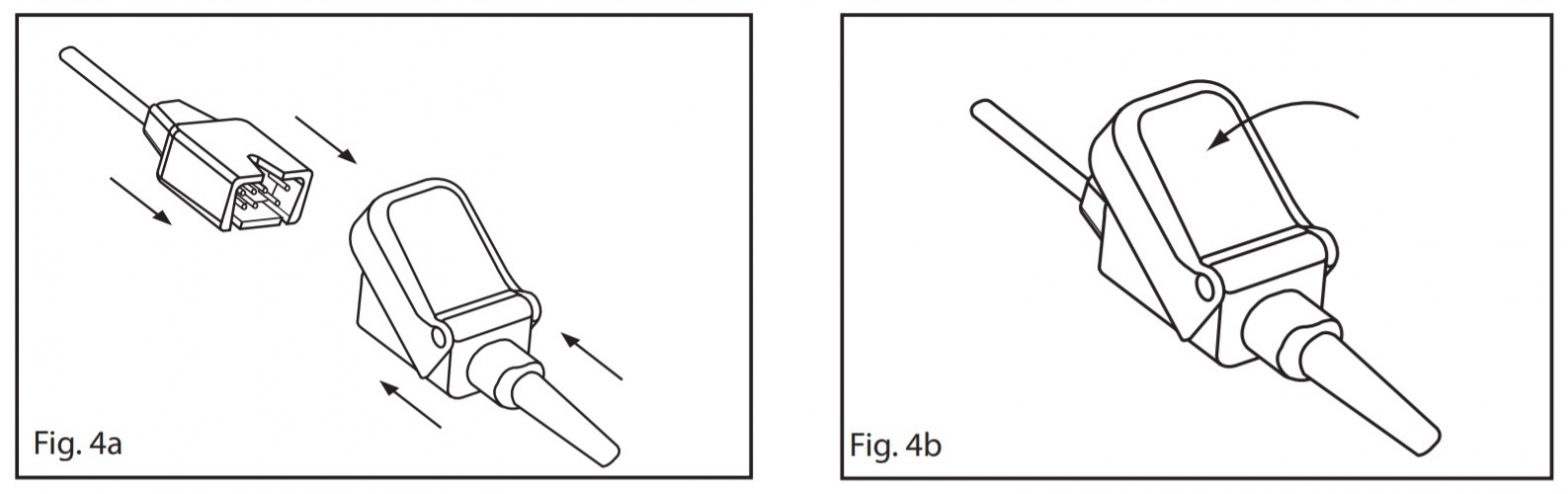
- The patient should turn their head to the opposite side from the ear where the sensor is attached. Attach the anchor tab on the cable to the anchor pad. Remove the release liner from the anchor pad. Secure the pad to the skin on the patient’s shoulder area. Refer to Fig. 3. Do not secure to the patient’s clothing.
C) Attaching the Sensor to the Patient CableLNCS
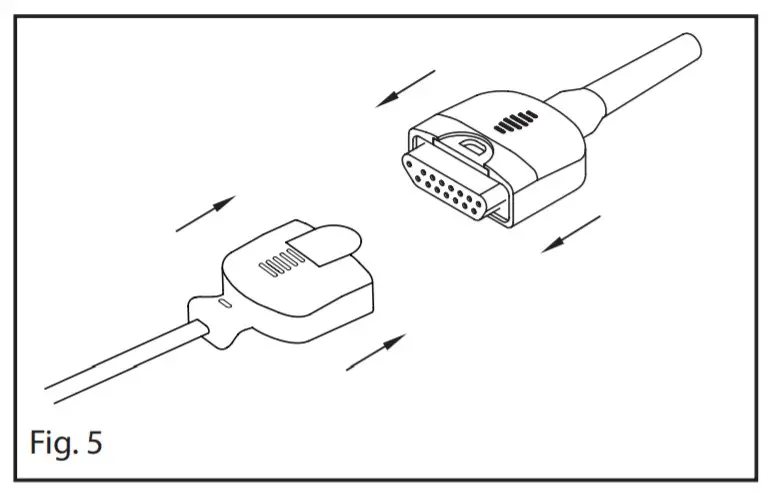
- Refer to Fig 4a. Properly orient the sensor and patient cable connectors and insert the sensor connectors completely into the patient cable connector.
- Refer to Fig 4b. Completely close the protective cover.
M-LNCS
- Refer to Fig 5. Orient sensor connector to patient cable connector as shown.
- Insert the sensor connector completely into the patient cable connector until it locks in place.
NOTE: When changing application sites, or reattaching the sensor, first attach the sensor to the application site, then connect the patient cable to the sensor.Stress and Exercise Testing
- Attach the E1 Ear Sensor to the patient following the steps in B (Attaching the Sensor to the Patient).
- Minimize unnecessary sensor motion during excessive patient movement. Using a headband, loop the ear sensor cable under the chin, and secure the cable under the headband on the side of the head opposite the ear sensor.
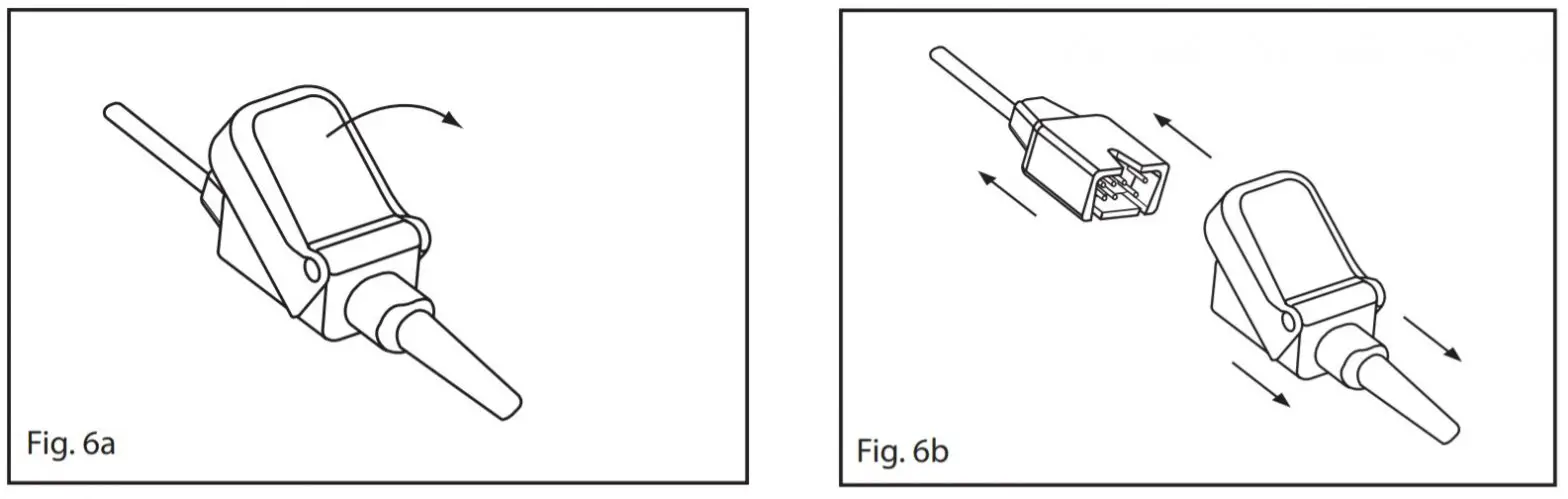
Disconnecting the SensorFrom the Patient Cable:LNCS
- Refer to Fig. 6a. Lift the protective cover to gain access to the sensor connector.
- Refer to Fig. 6b. Pull firmly on the sensor connector to remove it from the patient cable. To avoid damage, pull on the sensor connector, not the cable.
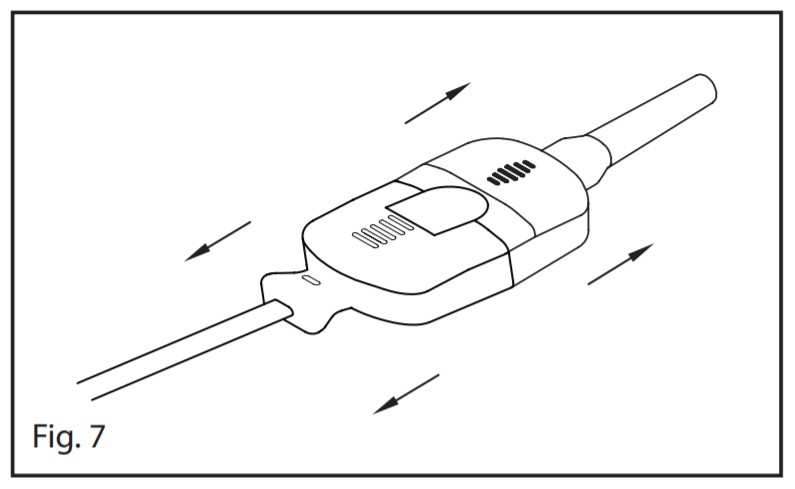
M-LNCS
- Refer to Fig 7. Pull firmly on the sensor connector to remove it from the patient cable. To avoid damage, pull on the sensor connector, not the cable.
From the Patient:
- Pull gently on the anchor pad and remove it from the patient’s shoulder.
- To remove the E1 Ear Sensor from the patient’s ear, gently pull apart the emitter and detector and remove.
CAUTION: To avoid discomfort to the patient or damage to the sensor, do not remove the sensor from the patient’s ear by pulling on the cable.CAUTION: Avoid positioning the patient so that external pressure is applied to the sensor at the measuring site.CAUTION: To prevent damage, do not soak or immerse the sensor in any liquid solution. Do not sterilize by irradiation, steam, autoclave or ethylene oxide.
SPECIFICATIONS
When used with Masimo SET pulse oximetry monitors, Masimo rainbow SET Pulse CO-Oximetry monitors, or with licensed Masimo SET pulse oximetry or Masimo rainbow SET Pulse CO-Oximetry modules, and patient cables, during no motion, the saturation accuracy of the E1 Ear Sensor from 70-100% is ± 2.5% (1 Std. Dev.). Pulse rate accuracy (from 25-240 bpm), during no motion, is ± 3 bpm (1 Std. Dev.) and low perfusion accuracy is ± 3 bpm (1 Std. Dev.).SpO2 accuracy was determined by testing on healthy adult volunteers in the range of 70% -100% SpO2 against a laboratory co-oximeter. Accuracy specifications are statistically distributed, and only about two-thirds of the measurements fall within the 1 Std. Dev. specification.
INSTRUMENT COMPATIBILITY


WARRANTYMasimo warrants to the initial buyer only that this product when used in accordance with the directions provided with the Products by Masimo, will be free of defects in materials and workmanship for a period of six (6) months. Single-use products are warranted for single-patient use only.THE FOREGOING IS THE SOLE AND EXCLUSIVE WARRANTY APPLICABLE TO THE PRODUCTS SOLD BY MASIMO TO BUYERS. MASIMO EXPRESSLY DISCLAIMS ALL OTHER ORAL, EXPRESS, OR IMPLIED WARRANTIES, INCLUDING WITHOUT LIMITATION ANY WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. MASIMO’S SOLE OBLIGATION AND BUYER’S EXCLUSIVE REMEDY FOR BREACH OF ANY WARRANTY SHALL BE, AT MASIMO’S OPTION, TO REPAIR OR REPLACE THE PRODUCT.WARRANTY EXCLUSIONSThis warranty does not extend to any product that has been used in violation of the operating instructions supplied with the product, or has been subject to misuse, neglect, accident, or externally created damage. This warranty does not extend to any product that has been connected to any unintended instrument or system, has been modified, or has been disassembled or reassembled. This warranty does not extend to sensors or patient cables that have been reprocessed, reconditioned, or recycled.IN NO EVENT SHALL MASIMO BE LIABLE TO THE BUYER OR ANY OTHER PERSON FOR ANY INCIDENTAL, INDIRECT, SPECIAL, OR CONSEQUENTIAL DAMAGES (INCLUDING WITHOUT LIMITATION LOST PROFITS), EVEN IF ADVISED OF THE POSSIBILITY THEREOF. IN NO EVENT SHALL MASIMO’S LIABILITY ARISING FROM ANY PRODUCTS SOLD TO THE BUYER (UNDER A CONTRACT, WARRANTY, TORT, OR OTHER CLAIM) EXCEED THE AMOUNT PAID BY THE BUYER FOR THE LOT OF PRODUCT(S) INVOLVED IN SUCH CLAIM. In no event shall Masimo be liable for any damages associated with a product that has been reprocessed, reconditioned, or recycled. THE LIMITATIONS IN THIS SECTION SHALL NOT BE DEEMED TO PRECLUDE ANY LIABILITY THAT, UNDER APPLICABLE PRODUCTS LIABILITY LAW, CANNOT LEGALLY BE PRECLUDED BY CONTRACT.NO IMPLIED LICENSE FORTHIS SINGLE-PATIENT SENSOR IS LICENSED TO YOU UNDER THE PATENTS OWNED BY MASIMO FOR SINGLE-PATIENT USE ONLY. BY ACCEPTANCE OR USE OF THIS PRODUCT, YOU ACKNOWLEDGE AND AGREE THAT NO LICENSE IS GRANTED FOR THE USE OF THIS PRODUCT WITH MORE THAN A SINGLE PATIENT.AFTER SINGLE-PATIENT USE, DISCARD THE SENSOR.PURCHASE OR POSSESSION OF THIS SENSOR CONFERS NO EXPRESS OR IMPLIED LICENSE TO USE THE SENSOR WITH ANY DEVICE WHICH IS NOT SEPARATELY AUTHORIZED TO USE E1 Ear SENSORS.
CAUTION: FEDERAL LAW (U.S.A.) RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. For professional use. See instructions for use for full prescribing information, including indications, contraindications, warnings, precautions, and adverse events.If you encounter any serious incident with the product, please notify the competent authority in your country and the manufacturer.The following symbols may appear on the product or product labeling:
|
SYMBOL |
DEFINITION | SYMBOL | DEFINITION | SYMBOL |
DEFINITION |
| Follow instructions for use |   |
Separate collection for electrical and electronic equipment (WEEE). | Caution: Federal law (USA) restricts this device is to sale by or on the order of a physician | ||
| Consult instructions for use | Lot code |
|
Mark of conformity to European Medical Device Directive 93/42/EEC | ||
| Manufacturer | Catalog number (model number) | Authorized Representative in the European community | |||
| Caution | Masimo reference number | Bodyweight | |||
| Use-by YYYY-MM-DD | Greater than | Storage temperature range | |||
| Do not re-use/Single patient use only | Less than | Keep dry | |||
| Non-Sterile | Storage humidity limitation | Do not use if the package is damaged and consult instructions for use | |||
| Not made with natural rubber latex | Fragile, handle with care | Atmospheric pressure limitation | |||
| Single patient – multiple uses | Medical device | Unique device identifier | |||
| Importer |   |
Distributor | Light Emitting Diode (LED) LED emits light when current flows through | ||
| Instructions/Directions for Use/Manuals are available in electronic format @ http://www.Masimo.com/TechDocs
Note: eIFU is not available in all countries. |
Patents: http://www.masimo.com/patents.htmMasimo, SET,![]()
![]()
PERFORMANCE SPECIFICATIONS: The table below shows Arms (Average Root Mean Square) values measured using the E1 Ear Sensor with Masimo SET Oximetry Technology in a clinical study.
| E1 Sensor | |
| SpO2 | Arms |
| 90-100% | 2.04 (2,04) % |
| 80-90% | 2.06 (2,06) % |
| 70-80% | 2.52 (2,52) % |
SaO2 versus error (SpO2 – SaO2) with linear regression fit and upper 95% and lower 95% limits of agreement





![]()
![]()
![]()
![]()
References
[xyz-ips snippet=”download-snippet”]

