Rapid COVID-19 Antigen Qualitative TestInstructions for Use
For prescription onlyFor in vitro diagnostic use onlyPlease read these instructions completely before beginning testing of specimens.
INTENDED USE
The Rapid COVID-19 Antigen Qualitative Test is a lateral flow colloidal gold immunoassay intended for rapid qualitative detection of nucleocapsid antigens from COVID-19 in human nasal swab, throat swab and sputum from individuals who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms.Results are for the identification of COVID-19 nucleocapsid antigen. Antigen is generally detectable in upper respiratory samples or lower respiratory samples during the acute phaseof infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses.The agent detected may not be the definite cause of disease.Negative results do not rule out COVID-19 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.The Rapid COVID-19 Antigen Qualitative Test is intended for use by trained clinical laboratory personnel in Point of Care (POC) settings.
PRINCIPLES OF THE PROCEDURE
This reagent is based on colloidal gold lateral flow immune assay.During the test, specimen extracts are applied to the test cartridge. If there were COVID19 virus nucleocapsid antigen in the extract, the antigen will bind to the COVID-19 monoclonal antibody. During lateral flow, the complex will move along the nitrocellulosemembrane toward the end of the absorbent membrane. Line T is coated with anotherCOVID-19 antibody, therefore, when the complex passes through the T-line, the complex is captured by T-line coating antibody resulting in coloring line or band on Line T.When the testing solution passes the Line C, colloidal gold-labeled non-COVID-19 antiIgG is captured by control Line C which is coated with non-COVID-19 IgG), results in coloring line or band on Line C.
REAGENTS
The following components are included in the Rapid COVID-19 Antigen Qualitative Test for rapid detection of COVID-19 kitMaterials Provided (Support for 25 Tests):
- Rapid OVID-19 Antigen Test Cartridge (25)
- Extraction Tubes (25)
- Extraction solution: 2 bottles/kit (enough for 25 tests)
- Instructions for use (1 copy)
Optional Materials:
- Screw-cap collection cup(25)
Materials Required but not provided:
- Nasal/Throat Swabs
- Timer
- Tube rack for specimens
- Any necessary personal protective equipment
WARNINGS AND PRECAUTIONS
- For in vitro diagnostic use.
- This test has been authorized only for the detection of antigen proteins of COVID-19, not for any other viruses or pathogens.
- Test results are determined by visual or other scanning tools.
- Proper specimen collection, storage and transport are critical to test performance.
- Other pathogenic microorganisms, including hepatitis and HIV virus, may be present in clinical specimens. Standard precautions and institutional guidelines should always be followed in handling, storing, and disposing of all specimens and all items contaminated.
- Dispose of used test kits as biohazardous waste in accordance with federal, state and local requirements.
- The COVID-19 external positive control have been prepared from in-activated recombinant viral proteins and do not contain infectious material.
STORAGE CONDITIONS & PERIOD OF VALIDITY
- Store extraction solution at 2-30℃, the shelf life is 12 months.
- Store the test cartridge at 2-30℃, the shelf life is 12 months.
- Test Cartridge should be used right after opening the pouch package.
SPECIMEN COLLECTION AND HANDING
Sputum Specimen Collection:
- Rinse the mouth with water.
- Expectorate deep cough sputum directly into a sterile, screw cap collection cup.
Nasal Swab Specimen Collection:
- Insert the swab into one nostril of the patient. The swab tip should be inserted up to 2.5 cm (1 inch) from the edge of the nostril.
- Roll the swab 5 times along the mucosa inside the nostril to ensure that both mucus and cells are collected
- Using the same swab, repeat this process for the other nostril to ensure that an adequate sample is collected from both nasal cavities. Withdraw the swab from the nasal cavity.
![]() Throat Swab Specimen Collection:
Throat Swab Specimen Collection:
Let the patient’s head tilt slightly, mouth open, and make “ah” sounds, exposing thepharyngeal tonsils on both sides. Hold the swab and wipe the pharyngeal tonsils on both sides of the patient with moderate force back and forth for at least 3 times.
Specimen Transport and Storage:
Samples should be tested as soon as possible after collection. Based on data available, specimens are stable for up to 24-hours at room temperature or 2° to 8°C.
TEST METHODS
The test should be operated at room temperature(15-30℃).
For Sputum Specimen
- Place the extraction tube with opening facing up. Press the extraction solution bottle to drip 10 drops of extract solution into the extractor tube without touching the edge of the tube.
- Vortex or thoroughly mix Sputum specimen. Do not centrifuge.
- Transfer 300 μL of specimen into the extractor tube using transfer pipette.
- Mix well and squeeze 10-15 times. Let stand for 2 minutes. Install the nozzle cap onto the extraction tube.
- Loading: Drip 2 drops of extraction solution into the sample well of the test cartridge and start the timer. If the 1st drop of sample solution on the test cartridge look very viscous and thick, add another drop of extraction solution instead of the sample solution.
- If the sample solution on the test cartridge still does not move after 10 minutes, dilute the sample solution further by adding 3 to 5 drops of the extraction solution, and repeat the last step on a new test cartridge.
- Read the results at 20~30 minutes. Positive results can be reported at 20 minutes; however, negative results must be reported after 30 minutes. If positive signal appears after 30 minutes, it should not be reported as positive.
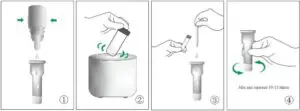

For Nasal Swab and Throat Swab Specimen
- Place the extraction tube with opening facing up. Press the extraction solution bottle to drip 6 drops of extract solution into the extractor tube without touching the edge of the tube.
- The extraction of specimen: Put the swab of collected specimen into the extraction tube, hold and press the swab head against the wall of tube with force while rotating the swab for about 10 seconds to release the antigen into the extraction solution from the swab head.
- If the swab is in the transport media, mix well the swab with media, then transfer 300 μL the media solution directly into the extraction tube from the Step 1.
- Removing swab: Squeeze the swab head while removing the swab in order to remove as much liquid as possible from the swab. Dispose of swabs according to biohazard waste disposal regulations.
- Install the nozzle cap onto the extraction tube.
- Loading: drip 2 drops of extraction solution into the samplewell of the test cartridge and start the timer.
- Read the results at 20~30 minutes. Positive results can be reported at 20 minutes; however, negative results must be reported after 30 minutes. If positive signal appears after 30 minutes, it should not be reported as positive.
INTERPRETATION OF TEST RESULTS
Line C must show a colored line or band to have a valid test result.
Valid results:
Negative result:
There is coloration or band on Line C only showing as following picture, suggesting that there is no COVID-19 antigen in the specimen.
Positive result:
There are coloration or bands on both Line C and Line T showing as follow pictures, suggesting that there is COVID-19 antigen in the specimen.
Invalid result:
There is no coloration or band on line C, as shown in the following pictures. The test is invalid or an error in operation occurred. Repeat the assay with a new cartridge.

REPORTING OF RESULTS
Positive Test:
Positive for the presence of COVID-19 antigen. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative Test:
Negative results are presumptive. Negative test results do not preclude infection and should not be used as the sole basis for treatment or other patient management decisions, including infection control decisions, particularly in the presence of clinical signs and symptoms consistent with COVID-19, or in those who have been in contact with the virus. It is recommended that these results be confirmed by a molecular testing method, if necessary, for patient management Control.
Invalid:
Do not report results. Repeat the test.
QUALITY CONTROL
- A procedure quality control is already integrated into the test. It appears as a red colored line or band in the control mark area if the test has performed correctly and reagents are reactive.
- External control standards (catalog number: MAK-Con-001) are also available for purchase by contacting the manufacturer directly if needed. The external controls are comprised of inactivated antigen and negative material.
- It is recommended that external controls are tested when A) The temperature for storage of the test kit falls outside recommended range. B) To verify higher than expected rate ofpositive or negative results. C) To investigate cause of repeated invalid results.
If the kit external controls do not perform as expected, do not report patient results. Contact Megna Health Technical Services 610-590-1768 or email [email protected].
LIMITATIONS OF THE PROCEDURE
- For best results, users should test specimens as quickly as possible after specimen collection.
- The amount of antigen in a sample may decrease as the duration of illness increases. Specimens collected after day 5 of illness are more likely to be negative compared to a RTPCR assay.
- Results from Rapid COVID-19 Antigen Qualitative Test should be correlated with the clinical history, epidemiological data, and other data available to the clinician evaluating the patient.
- Positive test results do not rule out co-infections with other pathogens.
- Negative test results do not eliminate the possible infection ofCOVID-19. A falsenegative test result may occur if the level of viral antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly.
- Negative test results are not intended to rule in other non COVID-19 viral or bacterial infections.
- Failure to follow the test procedure may adversely affect test performance and/or invalidate the test result.
- This device has been evaluated for use with human specimen material only.
- The rapid antigen test may fail to detect or detect with less sensitivity with mutations in the target epitope region of COVID-19 virus.
CLINICAL PERFORMANCE
The performance of the Megna Health Rapid COVID-19 Antigen Qualitative Test for rapid detection of COVID-19 antigen was established with 82 direct nasal swab, throat swab and sputum specimens prospectively or retrospectively collected and enrolled from individual symptomatic patients who were suspected of COVID-19. As with all antigentests, performance may decrease as days since symptom onset increases. 111 negative patients have been tested as well. Below is summary of the test results.
Test Performance within 7 Days of Symptom Onset

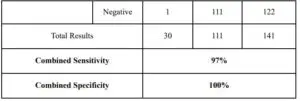
Test Performance Up to 24 Days of Symptom Onset


ANALYTICAL PERFORMANCE
Limit of Detection (Analytical Sensitivity)
The LOD for the COVID-19 Rapid Antigen Qualitative Test for rapid detection of COVID19 was established using limiting dilutions approach of inactivated COVID-19 virus material.The antigen material was spiked into clinical matrix, sputum, nasal swab and throat swabs, through step dilution process to 95% of all replicates test positive. The LOD in different sample matrix are summarized as below:
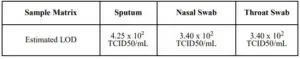
CROSS REACTIVITY (ANALYTICAL SPECIFICITY)
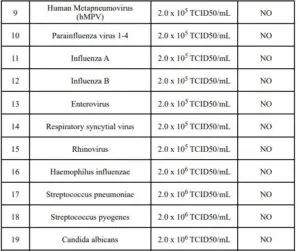
Cross-reactivity of the Rapid COVID-19 Antigen Qualitative Test for rapid detection of COVID-19 was evaluated by testing a panel of high prevalence respiratory pathogens that could potentially cross-react with the Rapid COVID-19 Antigen Test Kit. Each organism and virus was spiked into negative specimen of sputum or nasal swabs and tested in triplicates. The final concentration of each organism is documented in the following table.



Note :1 TCID50/mL≈0.7CFU/mlThese organism with concentration tested do not cross react with The Rapid COVID-19 Antigen Qualitative Test.
MICROBIAL INTERFERENCE STUDIES
The microbial interference for the Rapid COVID-19 Antigen Qualitative Test was performed using heat-inactivated COVID-19 antigen standard in the presence of various related pathogens, high prevalence disease agent and commensal organisms. This is todemonstrate the false negative do not occur when test for the known COVID-19 antigen.The concentration of standard antigen material is set at 3x of LoD and studied in human sputum or nasal swabs matrix. The final concentration of the organisms and virus are listed in the table below.


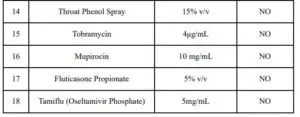
ENDOGENOUS INTERFERENCE SUBSTANCES STUDIES
Studies were performed to evaluate potential interference of substances that may be found in the lower respiratory tract on performance of the Rapid COVID-19 Antigen Test.
Results are listed below.


HIGH DOSE HOOK EFFECT
As part of the LoD study the highest concentration of heat-inactivated COVID-19 stock available was tested. There was no Hook effect detected.
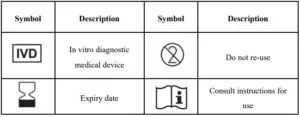
INDEX OF SYMBOLS

DEVICE TECHNICAL ASSISTANCE
For technical assistance, call Megna Health Technical Services at (855) 253-3634_or email at [email protected] or visit website at www.megnahealth.com.
GENERAL INFORMATION
Manufacturer
Megna Health Inc.436 Creamery Way, Suite 200Exton, PA 19341Phone: (855) 253-3634Email: [email protected]Website: www.megnahealth.comIssuing date: 2020-09-04

megna Rapid COVID-19 Antigen Qualitative Test – Instructions For Use – megna Rapid COVID-19 Antigen Qualitative Test – Instructions For Use –
[xyz-ips snippet=”download-snippet”]

