Anti-Human Globulin Anti-IgG(Rabbit)MTS™ Anti-IgG CardINSTRUCTIONS FOR USE
Anti-Human Globulin Anti-IgG(Rabbit)MTS™ Anti-IgG CardINSTRUCTIONS FOR USE
Intended Use
For in vitro diagnostic useFor Direct and Indirect Antiglobulin TestFor the detection of antigens to red blood cellsDoes not contain antibodies to complement componentsFor use with the ID-Micro Typing System™Observable IndicationsDrying, discoloration, bubbles, crystals, other artifacts, opened or damaged seals may indicate product alteration.Summary and Explanation of the TestAnti-Human Globulin was described in 1945 by Coombs, Mourant, and Race.Blood group antibodies of the IgG class, that were previously undetectable, reacted in the direct or indirect antiglobulin test (also known as the Coombs test). Anti-IgGreagents remain important tools for determining the presence or absence of IgG on human red blood cells. The reagent is used in the investigation of hemolytic disease of the newborn (refer to Limitations of the Procedure, item 12), transfusion reactions, and autoimmune hemolytic anemia in a direct antiglobulin test (DAT). The DAT detects IgG and/or C3 using either a polyspecific reagent solely or monospecific Anti-IgG and Anti-C3. Indirect antiglobulin tests are employed in compatibility testing, screening tests for donor and patient antibodies, antibody identification procedures, and antigen detection.Principles of the ProcedureThe combination of the antiglobulin reagent incorporated into gel, known as the ID-MTS™ Gel Test, was first described by Dr. Yves Lapierre. The Anti-Human Globulin Anti-IgG (Rabbit) MTS™ Anti-IgG Card can be used in both direct and indirect antiglobulin test systems to detect the presence or absence of IgG on human red blood cells.In the MTS™ Anti-IgG Card, red blood cells that are coated with IgG due to in vivo sensitization are detected with the direct antiglobulin test. The detection of in vitro sensitization is determined by the indirect antiglobulin test.The MTS™ Anti-IgG Card restricts the unbound IgG from moving through the gel during centrifugation. The unbound IgG does not neutralize the Anti-IgG incorporated in the gel.Red blood cells sensitized with IgG react with the Anti-IgG gel in the microtube during centrifugation. Strongly positive agglutination reactions produce a line of red blood cells layered at the top of the gel. Positive reactions will have varying degrees of visible red blood cell agglutinates suspended in the gel. Uncoated (unsensitized) red blood cells or red blood cells coated only with complement are not agglutinated by the Anti-IgG and will form a button at the bottom of the microtube. Some literature reports indicate that Anti-IgG reagents lacking anti-complement components occasionally fail to detect some antibodies. These antibodies are demonstrable only by the use of a polyspecific antiglobulin reagent. In some cases, those antibodies that are not detected by Anti-IgG may be clinically significant.ReagentsAnti-Human Globulin Anti-IgG (Rabbit) for the MTS™ Anti-IgG Card is prepared from pools of sera obtained from rabbits that have been immunized with human IgG. The serum is adsorbed to remove unwanted heterospecific antibodies and is suspended in a buffered gel solution. The reagent meets the present potency and specificity requirements of the FDA. Sodium Azide (0.1% final concentration) is added as a preservative.Anti-Human Globulin (Anti-IgG) suspended in a diluent and buffered gel solution is contained in the 6 microtubes of the MTS™ Anti-IgG Card.Contains: 6 tests per card
Precautions
- Do not use it beyond the expiration date.
- Do not freeze or expose cards to excessive heat.
- Do not use gel cards that have not been shipped in an upright position.
- Use reagents as furnished.Caution:All blood products should be treated as potentially infectious. Source material from which this product was derived was found negative when tested in accordance with current FDA-required tests. No known test methods can offer assurance that products derived from human blood will not transmit infectious agents.Caution:Sodium azide may be toxic if ingested and may react with lead and copper plumbing to form explosive compounds. If discarded into sink, flush with a largethe volume of water to prevent azide buildup.WARNING:Once a gel card is used in testing, it may contain infectious material and should therefore be handled and disposed of as biohazard waste.
- A clear liquid layer should appear on top of the opaque gel in each microtube. Do not use gel cards if the gel matrix is absent or the liquid level in the microtube is at or below the top of the gel matrix. Do not use gel cards that show signs of drying, discoloration, bubbles, crystals, or other artifacts. Do not use cards if foil seals appear damaged or opened.Note: Refer to the ID-Micro Typing System™ Interpretation Guide 9 for additional information related to the visual inspection of gel cards before use.
- Do not remove foil seal until ready to use. The foil should be removed immediately before testing or within 1 hour of testing. Once opened, the gel may begin to dry out which could affect test results (refer to Limitations of the Procedure, item 11).
- After removing the foil, visually inspect all gel cards to ensure that residual film does not block the opening of any microtube.Caution:The pipet tip should not touch the gel card. Erroneous results due to carryover may occur.
- Refer to the corresponding instructions for use of ORTHO™ Sera blood grouping reagents for further precautions when using those products.
- End User’s who choose to use commercial antisera in an off-label manner must ensure that the test method is appropriate by validating its intended use.
Specimen Collection, Preparation, and Storage
No special preparation of the patient is required prior to specimen collection. Collect all blood samples using acceptable phlebotomy techniques.Note:Refer to the corresponding instructions for use of ORTHO™ Sera blood grouping reagents for further precautions when using those products.Samples for Direct Antiglobulin Test (DAT)
- Samples intended for direct antiglobulin testing should be drawn into EDTA to prevent in vitro complement binding. If EDTA is unavailable, specimens drawn into ACD, CPD, or CPDA-1 are preferable to non-anticoagulated clotted specimens. Red blood cells should be tested within 24 hours after collection. Clotted samples should not be refrigerated. Some samples such as cord blood, blood stored for extended periods of time, or blood that has been incompletelyanticoagulated, may develop fibrin clots or particulates. The fibrin clots or particulates may interfere with the ID-MTS™ Gel Test and cause red blood cell entrapment at the top of the microtube. Testing should be repeated using red blood cells that have been washed to remove the clots or particulates.
INSTRUCTIONS FOR USE
Reagent Preparation
- Red blood cells that are stored for extended periods of time may become coated in vitro with complement and globulin proteins. Those samples coated with IgG will then test as DAT positive with this reagent.
- Rouleaux caused by serum or plasma with abnormally high concentrations of protein (such as in patients with multiple myeloma or Waldenstrom’s macroglobulinemia or from patients who have received plasma expanders of high molecular weight) may infrequently cause difficulties in the ID-MTS™ Gel Test interpretation.False-positive results or hazy reactions may occur with these samples but are rare. Samples exhibiting rouleaux should be washed several times insaline and retested. Laboratories are advised to consult their approved procedures.
- Hemolyzed and grossly icteric blood samples may cause difficulty in interpretation, and test results should be used with caution.
Samples for Indirect Antiglobulin Test
- Fresh serum or plasma collected with or without anticoagulants may be used in indirect antiglobulin procedures for antibody detection and identification. Testing should be performed as soon as possible. Samples that cannot be tested immediately should be stored at 2–8 °C or frozen. In the case of potential recipients of blood transfusion, there is an FDA requirement that the specimen should not be stored for longer than 3 days before testing. Antibodies dependent for their detection upon the binding of complement may not be detected if aged serum or plasma from an anticoagulated sample is used for antibody detection tests. Serum should be separated from the red blood cells when stored or shipped. Donor blood or commercial reagent red blood cells should be used within their dating period.
- Samples obtained from acid elution procedures can be used if properly neutralized and if centrifuged to remove any debris.
- Red blood cells that are direct antiglobulin positive should not be used in the indirect antiglobulin procedure.
- Hemolyzed and grossly icteric blood samples may cause difficulty in interpretation, and test results should be used with caution. Grossly lipemic samples containing particulates that clog the gel, as indicated by diffuse blotches of red blood cells in the microtube, may be clarified by centrifugation or filtration and retested.
- Rouleaux caused by serum or plasma with abnormally high concentrations of protein (such as in patients with multiple myeloma or Waldenstrom’s macroglobulinemia or from patients who have received plasma expanders of high molecular weight) may infrequently cause difficulties in the ID-MTS™ Gel Test interpretation. False-positive results or hazy reactions may occur with these samples but are rare. Samples exhibiting rouleaux should be washed several times in saline and retested. Laboratories are advised to consult their approved procedures.
- The use of enzyme-treated red blood cells with the MTS™ Anti-IgG Card may detect clinically insignificant antibodies. The MTS™ Buffered Gel Card is recommended when using enzyme-treated cells.
The MTS™ Anti-IgG Card is provided ready to use. Each microtube contains Anti-IgG suitable for one test. The gel card is heat-sealed with aluminum foil to preserve the integrity of the reagents. Variations in the liquid and/or gel levels between microtubes may normally be observed. However, do not use cards if the liquid level in the microtube is below the top of the gel matrix (refer to Precautions).
Testing Procedure
The procedures identified below are for manual testing only. When using automated instruments, follow the procedures that are contained in the operator’s manual provided by the device manufacturer. Laboratories must follow their approved validation procedures and are advised to consult the appropriate regulatory agencies to determine validation requirements. Refer to ID-Micro Typing System™ Interpretation Guide and ID-Micro Typing System™ Implementation Guide and Procedures for additional information.Materials ProvidedAnti-Human Globulin (Anti-IgG) suspended in a final diluent and buffered gel solution is contained in the 6 microtubes of the MTS™ Anti-IgG Card.Materials Required but Not ProvidedFor manual gel card processing:
- 3% ORTHO Pooled Screening Cells
- 0.8% ORTHO® Pooled Screening Cells
- 3% Selectogen® Reagent Red Blood Cells
- 0.8% Selectogen® Reagent Red Blood Cells
- 3% Surgiscreen® Reagent Red Blood Cells
- 0.8% Surgiscreen® Reagent Red Blood Cells
- 3% Resolve® Panel Reagent Red Blood Cells
- 0.8% Resolve® Panel Reagent Red Blood Cells
- ORTHO™ Sera blood grouping reagents– ORTHO™ Sera Anti-Fya– ORTHO™ Sera Anti-Fyb– ORTHO™ Sera Anti-S– ORTHO™ Sera Anti-s– ORTHO™ Sera Anti-D (IAT)– ORTHO™ Sera Anti-P1
- Quality Control Material is known to give the appropriate positive and negative test results for each reagent requiring quality control. Examples include, but are not limited to, Alba Q-Chek® Simulated Whole Blood Controls.
- MTS™ Diluent 2
- Pipets: 10 μL, 25 μL, and 50 μL
- Pipet Tips
- Test Tubes
- Dispenser pipet capable of delivering 1.0 mL
- Marking Pen
- ORTHO® Workstation
- ORTHO Optix™ ReaderFor automated gel card processing with the ORTHO VISION® Analyzer or ORTHO VISION® Max Analyzer:
- 0.8% ORTHO® Pooled Screening Cells
- 0.8% Selectogen® Reagent Red Blood Cells
- 0.8% Surgiscreen® Reagent Red Blood Cells
- 0.8% Resolve® Panel Reagent Red Blood Cells
- ORTHO™ Sera blood grouping reagents– ORTHO™ Sera Anti-Fya– ORTHO™ Sera Anti-Feb– ORTHO™ Sera Anti-S– ORTHO™ Sera Anti-s– ORTHO™ Sera Anti-D (IAT)– ORTHO™ Sera Anti-P1
- Alban-Chek® Simulated Whole Blood Controls
- MTS™ Diluent 2
- ORTHO VISION® Analyzer
- ORTHO VISION® Max Analyzer
Test ProcedureDirect Antiglobulin Test
- Bring samples and reagents to room temperature (18–25 °C).
- Visually inspect gel cards before use. Each microtube should have a clear liquid layer on top of opaque gel.Caution: Do not use gel cards if the gel matrix is absent or the liquid level in the microtube is at or below the top of the gel matrix. Do not use gel cards that show signs of drying, discoloration, bubbles, crystals, or other artifacts. Do not use cards if foil seals appear damaged or opened.Note: Refer to the ID-Micro Typing System™ Interpretation Guide for additional information related to the visual inspection of gel cards before use.
- Prepare a red blood cell suspension of approximately 0.8% in MTS™ Diluent 2 (e.g., deliver 1.0 mL of MTS™ Diluent 2 into a test tube and pipet 10 μL packed red blood cells into the diluent), mix gently.
- Label the gel card appropriately.
- Remove the foil seal from the MTS™ Anti-IgG Card or from the individual microtubes to be used for testing. After removing the foil, visually inspect all gel cards to ensure that residual film does not block the opening of any microtube.Note: Foil should be removed immediately before testing or within 1 hour of testing. Once opened, the gel may begin to dry out which could affect test results (refer to Limitations of the Procedure, item 11).
- Add 50 μL of red blood cells (cells must be diluted in MTS™ Diluent 2 to approximately 0.8% or be a commercial 0.8% red blood cell in a low ionic strength diluent specifically approved for use in the ID-Micro Typing System™) to each microtube.Caution: The pipet tip should not touch the gel card. Erroneous results due to carryover may occur.
- Centrifuge the prepared cards in the ORTHO® Workstation at the preset conditions installed by the manufacturer.
- After centrifugation, remove the gel card(s) from the centrifuge. Observe, read macroscopically the front and back of each microtube for agglutination and/or hemolysis, and record reactions. If either side of the microtube is positive, the reaction is to be considered positive. See Diagram 1.
Indirect Antiglobulin Test
- Bring samples and reagents to room temperature (18–25 °C).
- Visually inspect gel cards before use. Each microtube should have a clear liquid layer on top of the opaque gel.Caution: Do not use gel cards if the gel matrix is absent or the liquid level in the microtube is at or below the top of the gel matrix. Do not use gel cards that show signs of drying, discoloration, bubbles, crystals, or other artifacts. Do not use cards if foil seals appear damaged or opened. Note: Refer to ID-Micro Typing System™ Interpretation Guide 9 for additional information related to the visual inspection of gel cards before use.
- Label the gel card appropriately.
- Remove the foil seal from the MTS™ Anti-IgG Card or from the individual microtubes to be used for testing. After removing the foil, visually inspect all gel cards to ensure that residual film does not block the opening of any microtube.Note: The foil should be removed immediately before testing or within 1 hour of testing. Once opened, the gel may begin to dry out which could affect test results (refer to Limitations of the Procedure, item 11). Refer to the corresponding instructions for use of ORTHO™ Sera blood grouping reagents for further precautions when using those products.
- Add 50 μL of red blood cells (cells must be diluted in MTS™ Diluent 2 to approximately 0.8% or be a commercial 0.8% red blood cell in a low ionic strength diluent specifically approved for use in the ID-Micro Typing System™) to each microtube.Caution: The pipet tip should not touch the gel card. Erroneous results due to carryover may occur.
- Add 25 μL of serum or plasma to each microtube of the MTS™ Anti-IgG Card. The mixture may or may not touch the gel suspension.Caution: The pipet tip should not touch the gel card. Erroneous results due to carryover may occur.
- Incubate the MTS™ Anti-IgG Card for 15 minutes at 37±2 °C.Note: Incubation times in low ionic strength solutions between 5 minutes and 40 minutes have been recommended in the literature.14-16 No single incubation time will be optimal for all antibodies.
- After incubation, centrifuge the prepared cards in the ORTHO® Workstation at the preset conditions installed by the manufacturer.
- After centrifugation, remove the MTS™ Anti-IgG Cards from the centrifuge. Observe, read macroscopically the front and back of each microtube for agglutination and/or hemolysis, and record reactions. If either side of the microtube is positive, the reaction is to be considered positive. See Diagram 1.Note: If using ORTHO™ Sera blood grouping reagents approved for use with MTS™ Anti-IgG Card, follow that manufacturer’s instructions for use for the test procedure.
predation of Results
Refer to ID-Micro Typing System™ Interpretation Guide 9for additional information.Negative Result: No agglutination and no hemolysis of the red blood cells is a negative test result. Complete sedimentation of all red blood cells is present in the bottom of the microtube.Positive Result: Agglutination and/or hemolysis of the red blood cells is a positive test result. Red blood cells may remain suspended on the top of the gel or are dispersed throughout the gel in varying degrees. A few red blood cells may form a button in the bottom of the microtube in some positive reactions.
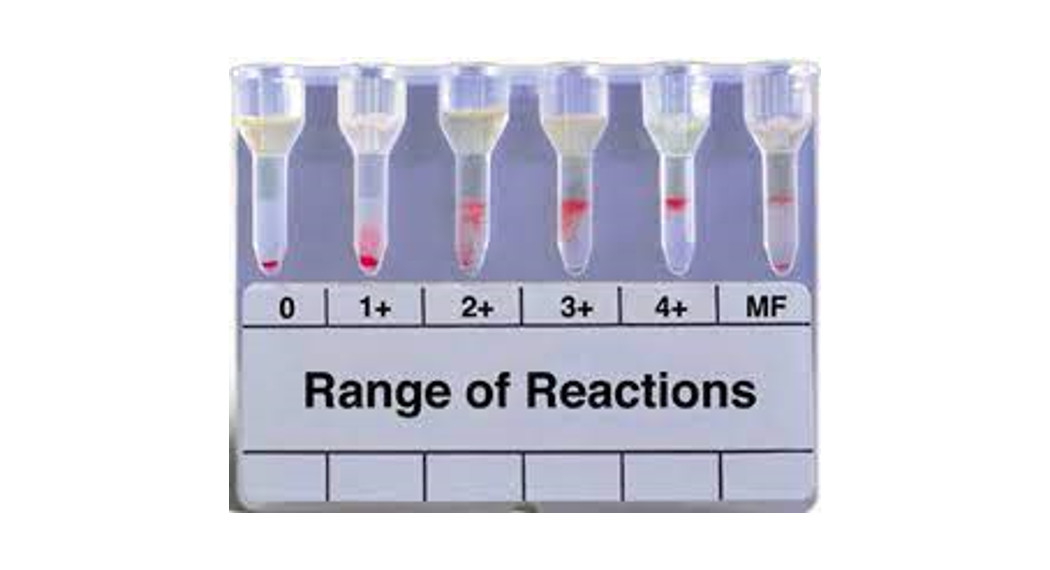
Stability of ReactionReaction Grading Guide (Use in conjunction with Diagram 1)
| 0 Negative | Unagglutinated red blood cells form a well-defined button at the bottom of the microtube. |
| it Reaction | Red blood cell agglutinates are observed predominantly in the lower half of the gel microtube. Unagglutinated red blood cells form a button in the bottom of the microtube. |
| 2+ Reaction | Red blood cell agglutinates are dispersed throughout the length of the gel microtube. Few unagglutinated red blood cells may be observed in the bottom of the microtube. |
| 3+ Reaction | The majority of red blood cell agglutinates are trapped in the upper half of the gel microtube. |
| 4+ Reaction | A solid band of red blood cell agglutinates on top of the gel. A few agglutinates may filter into the gel but remain near the predominant band. |
| Mixed Field | Red blood cell agglutinates at the top of the gel or is dispersed throughout the gel microtube accompanied by a button of negative red blood cells in the bottom of the microtube. See Note below. |
Note: Caution must be taken in interpreting a reaction as mixed field. Additional patient history and testing will be necessary for resolution. However, not all mixed cell situations have a sufficient minor population to be detected.Caution: Clots, particulates or other artifacts may cause some red blood cells to be entrapped at the top of the gel that may cause an anomalous result in a negative test (refer to Limitations of the Procedure, item 6).
Diagram 1: Examples of Reaction Grades

Stability of Reaction
For best results, it is recommended that reactions should be read immediately following centrifugation. Interpretations may be affected by the drying out of the gel, hemolysis of the red blood cells, and slanting of the reaction patterns due to storage in a non-upright position. Reactions stored in the refrigerator (2–8 °C) and effectively protected from evaporation were able to be interpreted for more than 14 days. Gel cards should not continue to be interpreted after the first sign of drying, or if red blood cell hemolysis is observed. The age and condition of the red blood cells, as well as the temperature at which the card is stored, will have an effect on how long gel cards can be interpreted before red blood cells will start to hemolyze. The presence of sodium azide in the gel may cause the red blood cells to become darker in color over time. This darkening does not interfere with the test result.
Quality Control
To confirm the specificity and reactivity of the MTS™ Anti-IgG Card, it is recommended that each lot be tested each day of use with known positive and negative antibody samples with the appropriate red blood cells. Reactivity must be present with the positive sample only.
Limitations of the Procedure
Refer to ID-Micro Typing System™ Interpretation Guide 9 for additional information.
- Strict adherence to the procedures and recommended equipment is essential.
- Proper centrifuge calibration is particularly important to the performance of the MTS™ Anti-IgG Card. The ORTHO® Max Analyzer have been exclusively designed to provide the correct time, speed, and angle. Workstation, ORTHO VISION ® Analyzer and ORTHO VISION®
- Red blood cells must be suspended in MTS™ Diluent 2 or be a commercial 0.8% red blood cell in a low ionic strength diluent specifically approved for use in the ID-Micro Typing System™.
- Variations in red blood cell concentration can markedly affect the sensitivity of test results. If red blood cell suspensions are too concentrated, they can give weaker results due to the increase in the antigen/antibody ratio. In addition, red blood cells may fail to completely migrate to the bottom of the microtube and could cause a false positive interpretation. When red blood cells are too low in concentration, they become difficult to visualize, and, in extreme cases, a weak positive can fail to be detected.
- False-positive or false-negative test results can occur from bacterial or chemical contamination of test materials, inadequate incubation time or temperature, improper centrifugation, improper storage of materials, or omission of test samples.
- Anomalous results may be caused by fresh serum, fibrin, or particulate matter in serum or plasma, or red blood cells that stick to the sides of the microtube. Anomalous results (i.e., a line of red blood cells on the top of the gel) may be observed with serum samples and can be minimized with the use of EDTA plasma.
- Red blood cells that test as DAT positive should not be used in an indirect antiglobulin procedure.
- The MTS™ Anti-IgG Card is not manufactured to detect Anti-C3 cell sensitizations. It may be used in the compatibility test; however, some literature reports indicate that the Anti-IgG may occasionally fail to detect antibodies that are demonstrable by the use of antiglobulin reagents containing Anti-C3.
- Optimal reaction conditions vary across antibody specificities. No single test method will detect all antibodies. In some low ionic strength test systems, certain antibodies, such as Anti-E and Anti-K, have been reported to be nonreactive.
- There is the potential for IgM antibodies to react in this test. Some patient antibodies that are IgM in nature may react with corresponding antigens in the upper portion of the microtube and be trapped in the top portion of the gel at the time of centrifugation resulting in a positive reaction.
- False-positive results may occur if a card that shows signs of drying is used in testing.
- Negative direct antiglobulin test results do not necessarily rule out hemolytic disease of the newborn (HDN), especially if ABO incompatibility is suspected.
- The Anti-H of Para-Bombay individuals may not be detectable in gel.
- Rouleaux caused by serum or plasma with abnormally high concentrations of protein (such as in patients with multiple myeloma or Waldenstrom’s macroglobulinemia or from patients who have received plasma expanders of high molecular weight) may infrequently cause difficulties in the ID-MTS™ Gel Test interpretation. False-positive results or hazy reactions may occur with these samples but are rare. Samples exhibiting rouleaux should be washed several times in saline and retested. Laboratories are advised to consult their approved procedures.
- Hemolyzed and grossly icteric blood samples may cause difficulty in interpretation, and test results should be used with caution.
- When using automated instruments, refer to the limitations contained in the operator’s manual provided by the device manufacturer.
Specific Performance Characteristics
Each lot of MTS™ Anti-IgG Card meets FDA requirements.The potency of Anti-IgG is verified by tests with red blood cells sensitized with decreasing amounts of Anti-D and Anti-Fy according to methods approved by the FDA. Additionally, each lot is tested with a known antibody to ensure Anti-IgG sensitivity of 0.1 IU/mL or greater.The absence of antibodies to C3 and C4 components has been confirmed by methods approved by FDA.The absence of contaminating heterophile agglutinins has been verified in tests employing group A1 B, and O red blood cells.For performance testing using ORTHO Sera blood grouping reagents, refer to the manufacturer’s specific instructions for use.Performance Characteristics on ORTHO VISION® AnalyzerMethod comparison testing was performed at five sites (four external and one internal site), that routinely perform immunohematology testing. Patient specimens were tested on the ORTHO VISION® Analyzer and the ORTHO ProVue® Analyzer. Individual microtube results were evaluated for agreement between analyzers. For microtube reaction grades to be in agreement between the analyzers, microtube reaction grades were either both negative or both positive (1+ through 4+) depending on the antibody being tested. The combined results from all sites are summarized in the following table.
| Test | Total | Positive | Negative | ||||||
| N | Agreement | LowerBound OneSided95% CI | N | %Agreement | LowerIndieBoundSided95% CI | N | (%)Agreement | Lower Bound One-Sided 95% CI | |
| AntibodyScreen | 11472 | 99.6% | 99.5% | 351 | 98.3% | 97.% | 11121 | 99.6% | 99.5% |
| Untreated andFicin treatedpanels | 2589 | 98.0% | 97.5% | 525 | 98.1% | 97.% | 2064 | 98.0% | 97.4% |
| DAT (IgG) | 557 | 98.6% | 97.4% | 105 | 100.0% | 97.% | 452 | 98.2% | 96.8% |
| IATCrossmatch | 990 | 97.4% | 96.4% | 465 | 100.0% | 99.% | 525 | 95.0% | 93.2% |
Agreement between two methods does not indicate which method gave the correct results.Performance Characteristics on ORTHO VISION® Max AnalyzerMethod comparison testing was performed at five sites (four external and one internal site), that routinely perform immunohematology testing. Patient specimens were tested on the ORTHO VISION® Max Analyzer and the ORTHOVISION® Analyzer. Individual microtube results were evaluated for agreement between analyzers. For microtube action grades to be in agreement between the analyzers, microtube reaction grades were either both negative or both positive (1+ through 4+) depending on the antibody being tested. The combined results from all sites are summarized in the following table.
| Test | Total | Positive | Negative | ||||||
| N | %Agreement | LowerBound OneSided95% CI | N | °A.Agreement | LowerIndieBoundSided95% CI | N | (%)Agreement | Lower Bound One-Sided 95% CI | |
| AntibodyScreen | 11356 | 99.8% | 99.8% | 479 | 98.3% | 97.0% | 10877 | 99.9% | 99.8% |
| Untreated andFind treatedpanels | 2537 | 99.8% | 99.5% | 675 | 99.6% | 98.9% | 1862 | 99.8% | 99.6% |
| DAT (IgG) | 563 | 99.5% | 98.6% | 180 | 98.9% | 96.5% | 383 | 99.7% | 98.8% |
| IATCrossmatch | 1024 | 99.9% | 99.5% | 466 | 100.0% | 99.4% | 558 | 99.8% | 99.2% |
Agreement between two methods does not indicate which method gave the correct results.Sample interpreted results were also evaluated for agreement between analyzers. For sample-interpreted results to be in agreement between the analyzers, interpretations were either both negative or both positive results. The combined results from all sites are summarized in the following table.
| Test | Total | Positive | Negative | ||||||
| N | %Agreement | LowerBound OneSided95% CI | N | %Agreement | Lower Bound One-Sided 95% CI | N | (%)Agreement | Lower Bound One-Sided 95% CI | |
| AntibodyScreen | 5108 | 99.8% | 99.6% | 314 | 98.4% | 96.7% | 4794 | 99.9% | 99.8% |
| AntibodyIdentification | 228 | 100.% | 97.9% | 126 | 98.2% | 96.3% | 102 | 100.0% | 97.1% |
Agreement between two methods does not indicate which method gave the correct results.Performance Characteristics on ORTHO Optix™ ReaderMethod comparison testing was performed at three sites (two external and one internal site), that routinely perform immunohematology testing. Individual microtube results were evaluated for agreement between ORTHO Optix™ Reader and the ORTHO VISION® Analyzer. For microtube reaction grades to be in agreement between the systems, microtube reaction grades were either both negative or both positive (1+ through 4+). Microtube results for a given test were combined across applicable ID-MTS™Gel Cards. The combined results from all sites are summarized in the following table.
References
| Test | Total | Positive | Negative | ||||||
| N | %Agreement | LowerBound OneSided95% CI | N | %Agreement | LowerIndieBoundSided95% CI | N | (%)Agreement | Lower Bound One-Sided 95% CI | |
| AntibodyScreen | 5084 | 99.98% | 99.9% | 175 | 100.0% | 98.3% | 4909 | 98.3% | 99.9% |
| UntreatedPanel Cells(Anti-IgG) | 1023 | 100.0% | 99.7% | 227 | 100.0% | 98.7% | 796 | 100.0% | 99.6% |
| IATCrossmatch | 1553 | 100.0% | 99.8% | 708 | 100.0% | 99.6% | 845 | 100.0% | 99.6% |
Agreement between two methods does not indicate which method gave the correct results.Note: For performance characteristics using ORTHO™ Sera blood grouping reagents, refer to the manufacturer’s specific instructions for use.Technical questions concerning these reagents should be directed to Ortho Care™ Technical Solutions Center at 1-800-421-3311.
References
- Coombs RRA, Mourant EE, Race RR. Detection of weak and “incomplete” Rh agglutinins. A new test. Lancet1945;ii: 15.
- Malyska H, Weiland D. The gel test. Laboratory Medicine 1994;25:81-85.
- Lapierre Y, Rigal D, Adam J, et al. The gel test: a new way to detect red cell antigen-antibody reactions. Transfusion 1990;30:109-113.
- Sherwood GK, Haynes BF, Rosse WF. Hemolytic transfusion reactions caused by failure of commercial antiglobulin reagents to detect complement. Transfusion 1976;16:417-420.
- Wright MS, Issitt PD. Anti-complement and the indirect antiglobulin test. Transfusion 1979;19:688-694.
- Howard JE, Winn LC, Gottlieb CE, et al. Clinical significance of the anti-complement components of antiglobulin antisera. Transfusion 1982;22:269-272.
- Issitt PD, Smith TR. Evaluation of Antiglobulin Reagents, In: A seminar on performance evaluation. Washington DC: American Association of Blood Banks 1976:25-73.
- Code of Federal Regulations; FDA, April 1, 2007; 21 CFR 606.151 (b).
- ID-Micro Typing System™ Interpretation Guide (J6902201), Ortho Clinical Diagnostics.
- Steiner EA, Casina TS, Mann NN, et al. Comparison of acid eluates tested in gel vs tube. Transfusion 1998; 38 (Supplement):37S.
- Novartis MCZ, Silveira EJ, Dorlhiac-Llacer PE, et al. Performance of acid elution test by conventional tube technique and gel test. Transfusion 2002;42 (Supplement):110S.
- Brecher M. (ed) Technical Manual, 16th Ed. Bethesda, MD: American Association of Blood Banks, 2008.
- ID-Micro Typing System™ Implementation Guide and Procedures (J6902200), Ortho Clinical Diagnostics.
- Jorgensen J, Nielsen M, Nielsen CB, et al. The influence of ionic strength, albumin and incubation time on the sensitivity of the indirect Coombs test. Vox Sang 1980;36:186-191.
- Moore HC, Mollison PL. Use of a low-ionic strength medium in manual tests for antibody detection. Transfusion 1976;16:291-296.
- Kosanke J, Dickstein B. Evaluation of incubation times using the ID-Micro Typing System. Transfusion 2002;42 (Supplement):108S.
- Yaskanin DD, Jakway JL, Ciavarella DJ. Red blood cell diluent composition is important for the detection of some anti-E. Immunohematology 2000;16:142.
- Merry AH, Thomson EE, et al. Quantitation of antibody binding to erythrocytes in LISS. Vox Sang 1984;47:125.
- Issitt PD. From a kill to overkill: 100 years of (perhaps too much) progress. Immunohematology 2000;16:18.
- Issitt PD. Applied Blood Group Serology, 4th Ed. Durham, NC: Montgomery Scientific Publications, 1998, p. 1182.
- Office of Biologics Research and Review, FDA. Recommended methods for Anti-Human Globulin Evaluation. Docket No. 84S-0182.
Glossary of Symbols
Revision History
| Date of Revision | Version | Description of Technical Changes• |
| 2020-11-12 | 7.0 |
|
* The change bars indicate the position of a technical amendment to the text with respect to the previous version of the document.Made under one or more of the following U.S. Patents:5,338,6895,460,9405,512,4325,863,8026,114,179Other Patents PendingMicro Typing Systems, Inc.an Ortho-Clinical Diagnostics Company1295 S.W. 29th AvenuePompano Beach, Florida 33069U.S. License No. 1177MTS, ID-MTS, and ID-Micro Typing System are trademarks of Micro Typing Systems, Inc.© Micro Typing Systems, Inc., 2008-2020Ortho Clinical Diagnostics
Version 7.0Pub. No. J32848_EN
[xyz-ips snippet=”download-snippet”]