
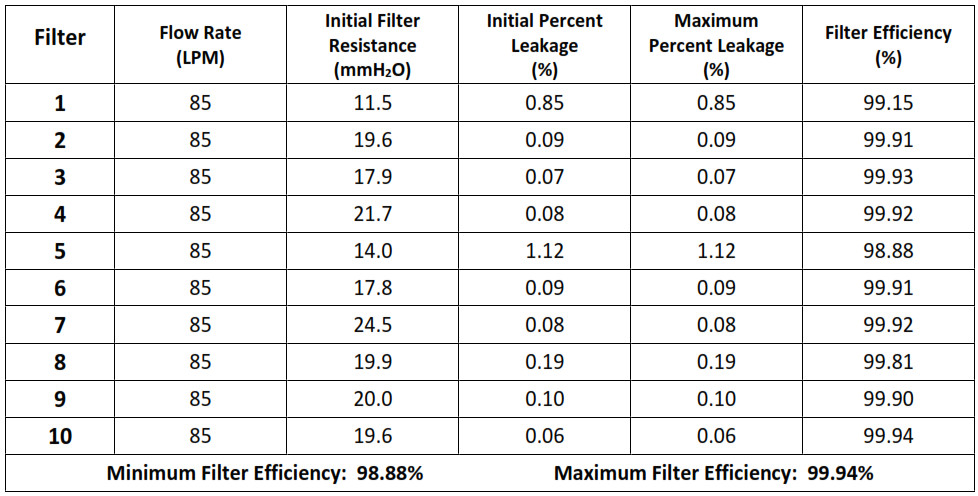
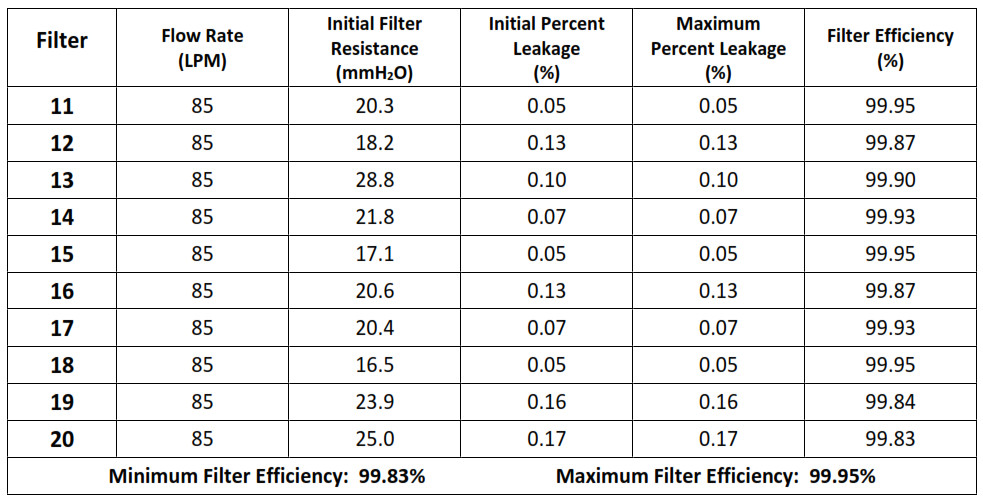
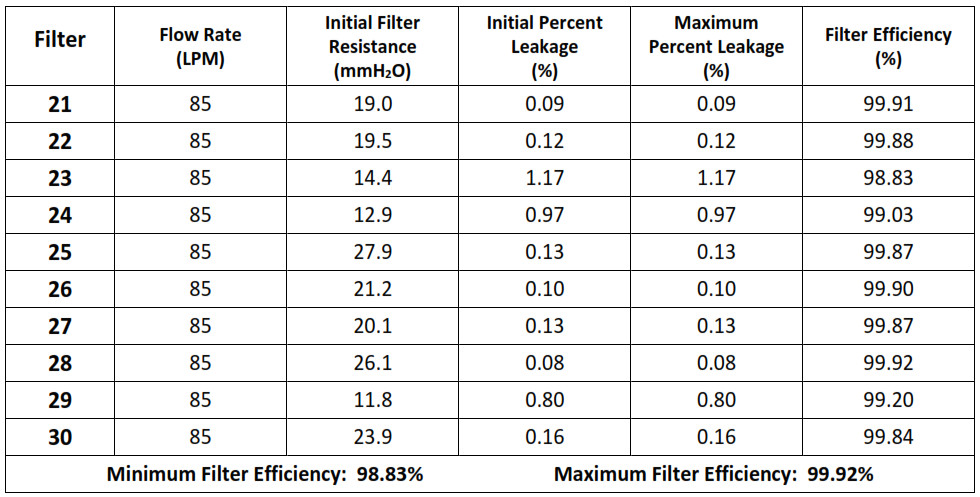
NPPTL COVID-19 Response: International Respirator AssessmentManufacturer: Chengde Technology Co., Ltd. Model Tested: KN95 Folding Protective Mask Date Tested: October 23, 2020These findings pertain to the Chengde Technology Co., Ltd., model KN95 Folding Protective Mask. The packaging and labeling for this product indicate that it meets GB2626-2006 (the Chinese standard for Respiratory Protective Equipment — Non-Powered Air-Purifying Particle Respirator).Thirty respirators were submitted for evaluation. The samples were tested using a modified version of NIOSH Standard Test Procedure (STP) TEB-APR-STP-0059. This modified assessment plan can be found here.No certificate of approval was provided with the samples received; therefore, the authenticity of the claims cannot be validated.The maximum and minimum filter efficiency was 99.95% and 98.83%, respectively. All thirty respirators measured more than 95%.While the above-listed product classification has similar performance requirements to NIOSH-approved devices, NIOSH does not have knowledge about the sustained manufacturer quality system and product quality control for these products. NIOSH also does not have knowledge about the product’s handling and exposures after leaving its manufacturer’s control.In addition, this product is an ear loop design. Currently, there are no NIOSH-approved products with ear loops; NIOSH-approved N95s have head bands. Furthermore, limited assessment of ear loop designs, indicate difficulty achieving a proper fit. While filter efficiency shows how well the filter media performs, users must ensure a proper fit is achieved.This assessment Is not a part of the NIOSH respirator approval process and will In no way lead to or preclude NIOSH approval through the official approval process. This assessment was developed as an assessment of the filter efficiency for those respirators represented as certified by an intemational certification authority, other than NIOSH, to support the availability of respiratory protection to US healthcare workers due to the respirator shortage associated with COVID-19. Only particulate filter efficiency was assessed.The results provided in this letter are specific to the subset of samples that were provided to NPPTL for evaluation.These results will be used to update the CDC guidance for Crisis Capacity Strategies (during known shortages).
Evaluation of International Respirators
Test: Modified TEB-APR-STP-0059Date Tested: October 23, 2020Report Prepared: October 23, 2020Manufacturer: Chengde Technology Co., Ltd.Item Tested: KN95 Folding Protective MaskCountry of Certification: China (GB2626-2006)
- The test method utilized in this assessment is not the NIOSH standard test procedure that is used for certification of respirators. Respirators assessed to this modified test plan do not meet the requirements of STD-0059. and therefore cannot be considered equivalent to N95 respirators that were tested to STP-0059.
- Respirators tested may not be representative of all respirators with the same certification mark NIOSH has no control over suppliers and distributors of respirators certified by other national or international parties.
- This assessment is not a confirmation that it conforms with any or all of its specifications in accordance with its certification mark.
- This assessment was not a part of the NIOSH approval program These results do not imply nor preclude a future approval through the NIOSH respirator approval program.
NPPTL COVID-19 Response: International Respirator Assessment








KN95 Folding Protective Mask User Manual – KN95 Folding Protective Mask User Manual –

