InnoSpire Deluxe
InnoSpire Deluxe

InnoSpire Deluxe

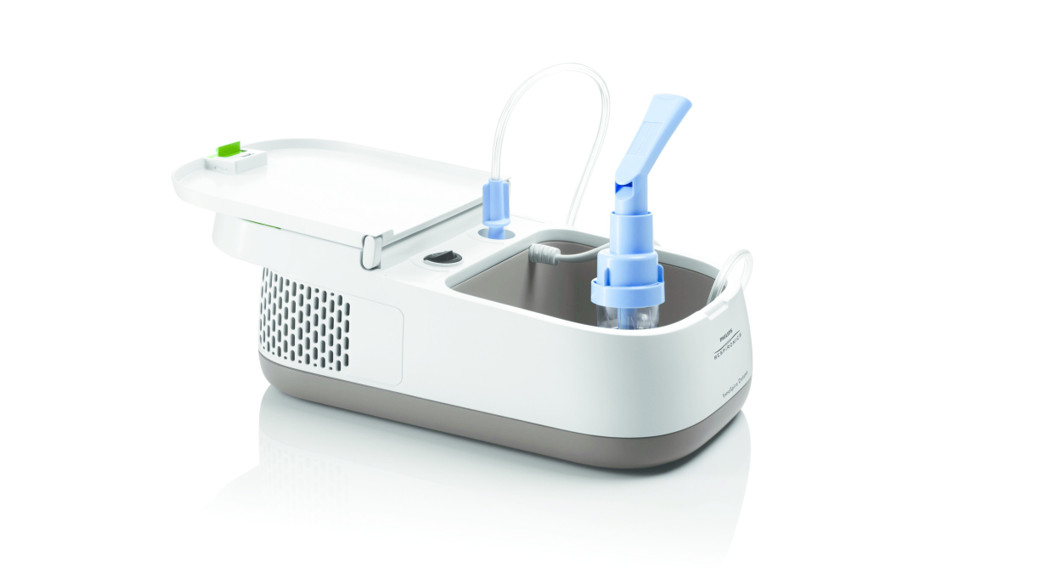
| 1. Nebulizer tubing | 7. Air filter (side view) |
| 2. Nebulizer mouthpiece | 8. Compressor air outlet |
| 3. Nebulizer handset | 9. Air inlet for filter (side view) |
| 4. Nebulizer holder (inside view) | 10. Lid |
| 5. On/Off power switch | 11. Storage compartment |
| 6. Compressor air openings | 12. Power cord location |
Instructions for use
InnoSpire DeluxeImportant: Read these instructions carefully before using this device. If you have any questions or problems with the product, please contact your healthcare provider or Philips Respironics Customer Service at +1 724 387 4000.
General information
Intended use: This product is intended to provide a source of compressed air for medical purposes. It is to be used with a pneumatic (jet) nebulizer to produce aerosol particles of medication for respiratory therapy for both children and adults.
 WARNINGS
WARNINGS
- Use the device for its intended purpose as described in this instruction manual as a compressor nebulizer system for aerosol therapy. Follow your doctor’s instructions. Any other use constitutes improper use and may be dangerous. The manufacturer cannot be held liable for any damage caused by improper, incorrect, or unreasonable use or if the unit is connected to electrical installations not complying with safety regulations.• It is recommended to have a backup device (e.g. MDI or battery-operated compressor) for respiratory delivery in case a situation arises when your nebulizer cannot be used (e.g. during a power outage or if your compressor becomes inoperable for any reason).• If the device has been outside of the operating conditions, allow to come back to normal operating specifications before use.• Any liquid spilled on or inside the unit should be allowed to dry before operating.
- Keep this manual for future reference.
- Use the product in a clean environment; one that is free from dirt, dust, pet hair, etc.
- device should not be used around flammable substances, or in the presence of a flammable anesthetic mixture with air, oxygen or nitrous oxide.
- No modification of this equipment is allowed.
- It is normal for the device to be warm to the touch when in use.
- Do not stack the compressor with other equipment.
- Electromagnetic information: Portable and RF communication devices such as cellular phones, pagers, etc. can interrupt the operation of electrical medical equipment. For this reason, your compressor must be placed far enough away from these devices to prevent interference. This device meets the IEC60601-1-2 standard for electromagnetic compatibility (EMC). EMC data sheets are available at the back of this document. EMC data sheets in other languages are available from Philips Respironics customer service at +1 724 387 4000. The CE mark on the product denotes compliance with all applicable EU Directives. Note that the Notified Body number does not apply to the RoHS (Restriction of the use of certain hazardous substances in electrical and electronic equipment) Directive.
- In case of failure and/or malfunction, read the “Troubleshooting” section. Do not open the compressor housing as this is not a serviceable product.
- The proper function of the device can be compromised if original replacement parts (e.g., filters) are not used.
- To reduce the risk of electric shock, DO NOT DISASSEMBLE.
- Indoor use only.
- Do not use the device without the filter.
- When using any electrical appliance, certain important safety measures must always be followed, including the following:• Use only manufactured-supplied original accessories and components;• Never submerge the unit in water as the device is not protected against water penetration;• Never touch the unit when your hands are wet or moist;• Do not leave the unit outdoors;• Place on a stable and horizontal surface when operating;• Make sure the air vent openings are not obstructed;• Do not allow unsupervised children or infirm individuals to use the unit;• Do not turn off the unit by simply pulling out the plug from the electrical outlet;• Do not use in a ventilator circuit;• Do not use if the device has cracks or is damaged.
- Make sure that the electrical rating shown on the rating label on the bottom of the unit corresponds to your main voltage and frequency before plugging in the device.
- Do not use any adapter, single or multiple, and/or extension cord.
- Do not leave the unit plugged in when not in use. Unplug the device from the electrical outlet when not in operation.
- Follow the manufacturer’s instructions for installing the device. The manufacturer cannot be held responsible for any damage caused by incorrect installation.
- The PO power supply cord cannot be replaced by the user. In case the power supply cord becomes damaged, contact Philips Respironics customer service for replacement (there is no service option).
- Do not overfill the storage compartment with accessories.
- Beef ore performing any cleaning operation or filter replacement, disconnect the device from the main supply by unplugging it.
- Small parts can be inhaled, swallowed or dangerous. Cable, due to its length, may result in provoking strangulation or asphyxiation. Do not leave the device alone with a small child or physically challenged individual or an individual with learning difficulties.
- If you no longer use the device, dispose of it according to the local regulations in force.
- Remember to:• Use this device only with medications prescribed by your doctor;• Use the accessories recommended by your doctor.
Instructions for use
Remove the compressor from the carrying case if supplied before use.
- After unpacking the device, check the compressor and accessories for visible damage or defects such as cracks in the plastic housing which could expose electrical components. Contact Philips Respironics customer service or your product distributor to report any damaged product.
- When taking a treatment, sit upright and relaxed.
- Plug the compressor into the electrical outlet.
- Refer to the nebulizer and accessory instructions for use before treatment.
- To start the treatment, switch the device ON by pressing the ON / OFF switch onto the “I” position.
- When the treatment has been completed, switch the unit OFF by setting the switch position to “O” and disconnect the plug from the electrical outlet.
- Do not close the lid when the nebulizer tubing is attached.
Cleaning and maintenance
![]() During cleaning, make sure the power cord is not plugged in the electrical outlet.Cleaning of the compressor, nebulizer, and accessoriesUse a damp cloth to wipe down the outside of the compressor at least once each month. Refer to the nebulizer and accessory instructions for use for cleaning of the accessories and nebulizer.
During cleaning, make sure the power cord is not plugged in the electrical outlet.Cleaning of the compressor, nebulizer, and accessoriesUse a damp cloth to wipe down the outside of the compressor at least once each month. Refer to the nebulizer and accessory instructions for use for cleaning of the accessories and nebulizer.
Replacement of the nebulizerThe nebulizer and other accessories should be replaced according to its instructions.Replacement of the filterThe filter is to be replaced when it appears discolored or wet.
Troubleshooting
The device does not turn on
- Make sure the plug is firmly fitted to the electrical outlet.
- The thermal protector may have been activated for the following reasons:a) the device was used near a heater or in a hot environmentb) the air vent openings (6) are obstructed and do not allow for the motor ventilationIf this is the case set the ON/OFF switch to ‘O’ and let it sit for at least 60 minutes. In the event of a malfunction (insufficient pressure and flow) turn the unit off for 10 seconds and turn it back on.
After each cleaning, check that the unit is working properly by verifying that air still comes out of the nozzle when the unit is turned to the “ON” position.Unauthorized repair voids warranty.If the device does not work properly after attempting these troubleshooting steps, contact your authorized product distributor or Philips Respironics Customer Service at +1 724 387 4000.Maintenance and repairsNever open the device. There are no serviceable parts within the unit. The compressor requires no lubrication or maintenance.
Technical specifications
| Electricity supply | 230V/50Hz. 1 Amp |
| Thermal overload protector | Resettable thermal fuse; functioning temperature 150 C° |
| Compressor max. pressure | 360 kPa (3.6 bar) |
| Average flow rate | 7 LPM 70 kPa (0.7 bar) |
| Max flow rate | 9.8 PM |
| Weight | 2.2kg |
| Size | 380mm x 170mm 110mm |
| Noise level | 58±3dBA |
Technical specifications
| Electricity supply | 220V/60Hz. 1.2 Amp |
| Thermal overload protector | Resettable thermal fuse; functioning temperature 130 C° |
| Compressor max. pressure | 268 kPa (2.68 bar) |
| Average flow rate | 6 LPM 70 kPa (0.7 bar) |
| Max flow rate | 10.7 PM |
| Weight | 2.0kg |
| Size | 380mm x 170mm x 110mm |
| Noise level | 58±3dBA |
Class II device, T ype BF device (device with specific protection against electrical hazards).DOUBLE INSULATED
Reference to standards
| Electric safety standards IEC 60601-1 |
| Electromagnetic compatibility according to IEC 60601-1-2 |
Ambient conditions
| Storage conditions | |
| T emperature | MIN -25 °C – MAX 70 °C (-13 °F – 158 °F) |
| Humidity | MIN 10% RH – MAX 95% RH |
| Operating conditions | |
| T emperature | MIN 5 °C – MAX 40 °C (41 °F –104 °F) |
| Humidity | MIN 10% RH – MAX 95% RH |
| Operatingatmospheric pressure | Performance may vary depending on altitude above sea level, barometric pressure, and temperature |
Symbol glossary
| ON (power) | ATTENTION | Class II, double insulated | |||
| OFF (power) | Alternating current | T ype BF applied parts | |||
| Serial number | Separate collection | Indoor use only | |||
| Follow instructions for use | Manufacturer | T emperature limitation | |||
| Atmospheric pressure | Humidity |
IP21 Housing Ingress Protection rate: IP21 (protected from the penetration of solid bodies with dimensions greater than 12 mm. Protected from the penetration of vertically falling water drops).
Warranty
Respironics, Inc warrants the compressor to be free from defects in materials and workmanship under normal use and operation for a period of 5 years from the date of purchase from Respironics, Inc. The warranty is limited to repair or replacement, at Respironics, Inc’s sole option, of any such component or equipment claimed to be defective when a claim is shown to be bona fide by evaluation by Respironics, Inc. This warranty does not extend to any components or equipment subjected to misuse, improper operation, accidental damage, or unauthorized repairs, and is not extended to charges of, or for, labor repairs. All items returned must be properly packaged and shipped, prepaid, by the product distributor servicing the unit. Respironics, Inc shall not be liable to the purchaser or others for the loss of use of equipment or for indirect, or incidental or consequential damages thatmight arise.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
References
[xyz-ips snippet=”download-snippet”]