ISOCUBE SS Disposable Isolette Bag InstructionsPrep Tech, LLC1614 Wolf CircleLake Charles, LA 70605preptechmed.com
Instructions for Healthcare Providers and Facilities: ISOCUBE™ SS
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for ISOCUBE SS, for use by healthcare providers (HCP) as an additional layer of barrier protection in addition to personal protective equipment (PPE) to prevent HCP exposure to pathogenic biological airborne particulates by providing temporary isolation of hospitalized patients with suspected or confirmed diagnosis of COVID-19, at the time of definitive airway management, or when performing airway-related medical procedures, or during certain transport of such patients during the COVID-19 pandemic.
Authorized non-transport use of ISOCUBE SS is only for definitive airway management (e.g., intubation, extubation and suctioning airways), when performing any airway-related medical procedures (e.g., high flow nasal cannula oxygen treatments, nebulizer treatments, manipulation of an oxygen mask or continuous positive airway pressure/bi-level positive airway pressure [CPAP/BiPAP] mask use, airway suctioning, percussion and postural drainage), or during certain patient transport. Authorized use of ISOCUBE SS during patient transport is only within a hospital setting for temporary transfer with direct admission within the hospital in the presence of a registered nurse or physician. The patient must have constant monitoring of vital signs, electrocardiogram (EKG), oxygen saturation (SpO2%), end-tidal carbon dioxide (EtCO2) if available throughout transport. For all authorized uses, the patient must always have supplemental oxygen during the use of ISOCUBE SS. Limit the duration of transport to 30 minutes if end-tidal CO2 monitoring is not available with an inline blower fan on and under direct observation.
ISOCUBE SS has not been FDA-approved or cleared for this use; ISOCUBE SS has been authorized for emergency use by FDA under a EUA. ISOCUBE SS has been authorized only for the duration of the COVID-19 public health emergency declaration that circumstances exist justifying the authorization of the emergency use of medical devices under section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1) unless the declaration is terminated or authorization is revoked sooner.
HCP must follow these instructions, as well as procedures at their healthcare facility to use ISOCUBE SS. The instructions below are to assist in using ISOCUBE SS. ISOCUBE SS is an adjunctive protective barrier designed to mitigate risk to HCP. ISOCUBE SS is not meant to be a stand-alone unit of PPE. ISOCUBE SS must always be used with appropriate PPE and pursuant to the guidance of your institution.
The instructions below are to assist in using ISOCUBE SS. ISOCUBE SS is an adjunctive protective barrier designed to mitigate risk to HCP. ISOCUBE SS is not meant to be a stand-alone unit of PPE. ISOCUBE SS must always be used with appropriate PPE and pursuant to the guidance of your institution.
All connections must be tightly secured and checked frequently. Any time anyone is within ISOCUBE SS, direct observation is required. Inspect ISOCUBE SS prior to use. Any wear/tear of the chamber or other signs of degradation on ISOCUBE SS must promptly be reported to Prep Tech, LLC. The healthcare facility must not use on patients and must dispose of such an ISOCUBE SS. Rx Only.
WARNINGS:
- The flammability of ISOCUBE SS has not been tested. No interventions that could create a spark or be a flammable source must be used within ISOCUBE SS.
- Remove ISOCUBE SS and use the standard of care if there is difficulty visualizing or identifying anatomic landmarks or inability to intubate after the first try.
- Prolonged use of ISOCUBE SS may induce hypercarbia in a spontaneously breathing patient. ISOCUBE SS must be used with medical airflow and suction both on and working, under direct observation, and with EtCO2 monitoring if available.
- Patient transport must only occur within a hospital setting for temporary transfer with direct admission within the hospital in the presence of a registered nurse or physician. Maintenance of negative pressure with adequate airflow must be assured. All patients must be on supplemental oxygen. Patients must have continuous monitoring of SpO2%, vital signs, EKG, and EtCO2 if available during transport.
- Use caution prior to using on non-sedated or lightly sedated patients with severe claustrophobia and/or confined space anxiety.
- When using ISOCUBE SS, patients must always be receiving supplemental oxygen.
- Patients with diminished hearing may have difficulty understanding the provider while inside ISOCUBE SS.
- The ISOCUBE SS clear negative pressure isolette chamber is single-use and must be disposed of following the disposal instructions after use.
- The ISOCUBE SS base and rails are multi-use and must be sanitized between patient usage.
- If ISOCUBE SS is accidentally folded or the air-ports are blocked, this may result in patient injury.
- Delayed emergency removal of the device may result in patient injury.
CONTRAINDICATIONS:
ISOCUBE SS is not authorized for use on:
- Patients needing emergent endotracheal intubation with severe hypoxemia
- Patients with an anticipated or known history of difficult airway
- Patients with other anatomical abnormalities that might interfere with clinical care including decreased neck mobility from arthritis or other causes
- Patients with communication disorders that might interfere with clinical care
- Children under 45 lbs
- Patients with an anticipated or known history of claustrophobia
- Bariatric patients
- Patients with uncontrolled movements that may prevent the patient from being able to remain enclosed in the tent enclosure
- Patients in elderly care centers (non-hospital environment)
- Patients in ambulance transport.
ISOCUBE™ SS INSTRUCTIONS FOR USE
Device Description:ISOCUBE SS is a multi-use base and rail system with a single-use, clear, negative pressure chamber that attaches to a standard hospital or surgical bed, or stretchers and extends around the patient’s head, neck, and shoulders. Access holes with four (4) integrated gloves are built into the chamber to allow for isolated patient access. The negative pressure environment is generated via wall-mounted hospital vacuum lines or 1-2 negative pressure pumps equipped with in-line high-efficiency particulate air (HEPA) filter(s). The ISOCUBE SS unit acts as an added layer of a physical barrier in addition to PPE to prevent HCP exposure to pathogenic biological airborne particulates. ISOCUBE SS will be available to HCP and healthcare facilities.Items You Will Need:
- ISOCUBE SS Device
- 1 or 2 Facility Wall-Mounted Pressure Regulators (capable of maintaining continuous high suction at 200mmHg to produce 30 LPM per line) OR Portable Vacuum Pump(s) (capable of maintaining continuous high suction at 200mmHg to produce 30 LPM per line) with Inline HEPA Filter(s) (single use only; 0.3 µm or better filtration)
- 1 Facility Wall-Mounted Oxygen Delivery Regulator
- 1 or 2 Healthcare Facility Standard Suction Hose Lines (minimum 1/4″ID) (Single Use Only)
- 1 Healthcare Facility Standard Oxygen Line (usually 3/16″ID) (Single Use Only)
- 1 Blanket for Patient
- Endotracheal tube
- Nasal cannula
- Oxygen mask
ISOCUBE SS Device Components:
- (1) ISOCUBE SS Stainless Steel Base
- (2) ISOCUBE SS Stainless Steel Rails
- (1) ISOCUBE SS Clear Plastic Isolette Negative Pressure Chamber

Setup Instructions
- Remove all ISOCUBE™ SS components from the shipping box. Peel away/discard any protective plastic/paper to expose all stainless steel. Disinfect stainless steel before use as required by organizational policy (see sanitation section at the end of this document).
- Remove the isolette (has foam and white tape border) and drape from packaging. Set small folded drape aside.
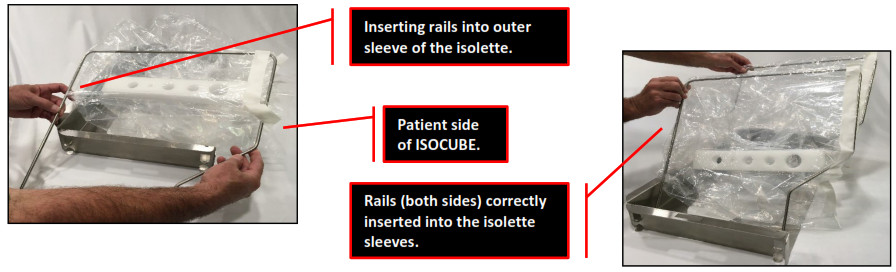
- With rail “bends” facing the open/patient side of the base, individually insert both rails into each of the outer sleeve cavities of the isolette, being careful not to tear the plastic.

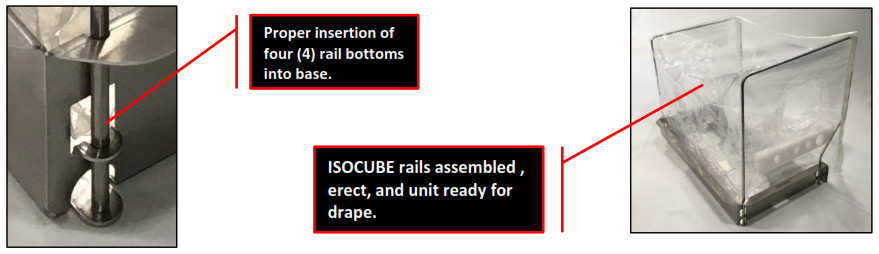
- Slide the four (4) rail ends completely down to the metal stops. The isolette should be erect and taut.

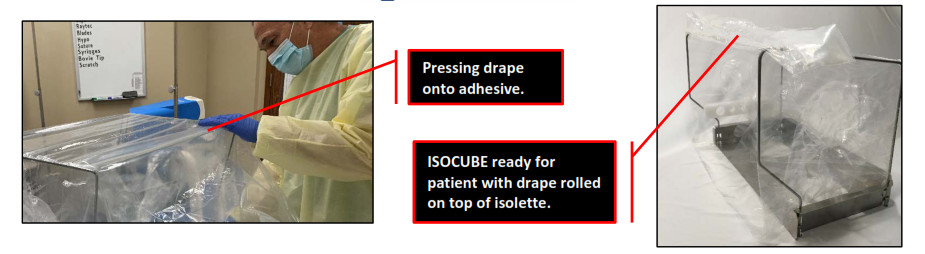
- Completely remove all white tape on both sides and top of ISOCUBE to expose adhesive.
- Unfold the drape. With the long side of the drape folded in half, press the long drape edge along with the adhesive starting on the top of ISOCUBE, then adhere the drape to the sides (drape sides will extend past the bottom of ISOCUBE).
- Roll adhered drape onto top of ISOCUBE ready to be unfurled onto the patient.

Preparing ISOCUBE™ SS for a Patient
- Place ISOCUBE above or below the bed mattress. Gather all instruments/tools needed to perform a planned medical procedure and place them inside ISOCUBE toward the sides (leaving room in the center for a patient’s head).

-
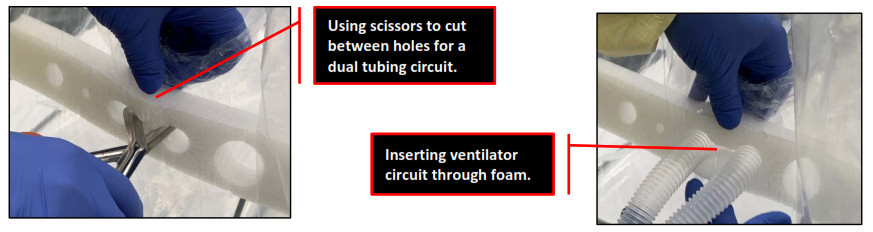
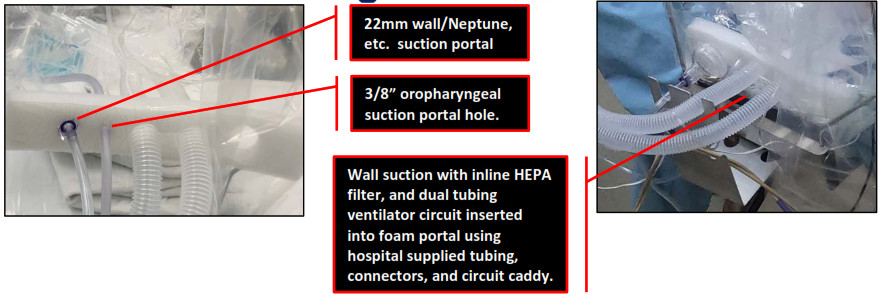
Route the oxygen delivery system (anesthesia, ventilator circuit tubing with EtCO2 tubing, Ambu bag, etc.) through the white foam tubing portal of ISOCUBE. Perforated holes are provided for single or dual tubing circuits. The largest hole fits a standard single tubing circuit. If using a dual tube system, use scissors to cut a slit between the foam holes before inserting the tubing.

- Insert the end of one or more vacuum sources (wall suction, Neptune, etc.) through the far left (22m) foam port.
- Route the oropharyngeal suction tubing into ISOCUBE through the second to the left hole (3/8”), leaving ample length needed for suctioning the airway. Leave the oropharyngeal suction turned on to optimize vacuum flow.

Applying the Drape to a Patient
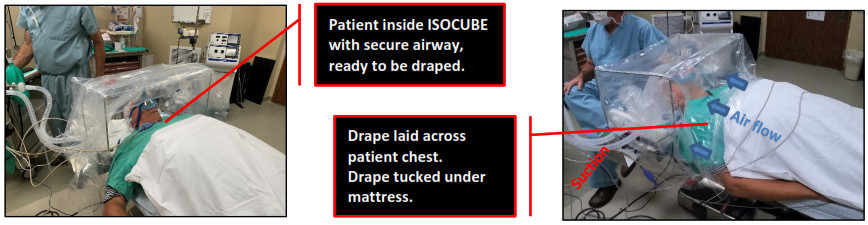
- The patient’s head should be inserted into ISOCUBE a few inches from the HCP side of the base. Once inside, quickly secure the patient’s airway for the appropriate medical procedure (CPAP, BiPAP, etc.).
- Unfurl the drape and lay the long end across the patient’s chest. Tuck the drape ends under the bedside mattress.
Notes:
- Room air is drawn into ISOCUBE (blue arrows) under the drape edge and sides then out through filtered suction.
- Continuous drawn air through hospital-supplied suction (red) is required to generate negative pressure and to remove aerosolized droplets/particles that may contain harmful viruses or bacteria.

Emergency Access to Patients inside ISOCUBE™ SS

- Opening ISOCUBE in an emergency can potentially expose HCP’s to aerosolized droplets contained within ISOCUBE
- ALWAYS wear PPE using ISOCUBE
- If direct access to the patient is needed, lift the drape while tucking it around the back of the patient’s head.
- If more access is needed, use scissors or a knife to cut open the isolette or remove it completely as depicted in the next section of this document.
Emergency/Removal of ISOCUBE™ SS
- Have an alternative airway circuit on hand ready to put on the patient.
- Slide the ISOCUBE away from the patient while keeping the loose drape end close to the patient’s head.
- While keeping the drape attached to ISOCUBE, tuck the drape under the ISOCUBE base and place it on a nearby cart or table.The isolette may contain harmful particles. Keep the isolette sealed as much as possible.
- Secure a new airway for the patient and prepare to dispose of the isolette.

ISOCUBE™ SS Disassembly

- ISOCUBE™ SS clear plastic isolette is used once and then discarded.
- ISOCUBE™ SS base and rails are sanitized and reused.
- The ISOCUBE after use is a biohazard and requires proper disposal per facility standard procedures.
- Full PPE must be worn at all times during disposal of ISOCUBE’s isolette following the facility’s policy and protocol for the disposal of biohazard waste.
- Place contaminated items such as ventilation/suction tubing used during the medical procedure inside the isolette.
- Remove rails by lifting them up and out of their slots. Lay the rails (still inside their slots) on the collapsed isolette.
- Remove the rails from the isolette side pouches and set them aside (or under the isolette on the base).
- Fold the isolette and all contaminated items into a large wad, using the “room-exposed side of the drape” on the outside of the wad.
- Dispose of all items following the facility’s policy and a proper protocol for disposal of biohazard waste.

ISOCUBE™ SS Sanitation
- For cleaning, dispense a towelette of Cavicide wipe or other hospital-approved EPA-registered quaternary ammonium compound/isopropyl alcohol-based hospital disinfectant wipe and wipe clean the inner and outer surfaces of the Base and Rails to remove any soil.
- If visible soil remains, repeat the procedure. Let all parts sit visibly wet for at least 2 minutes. Allow all parts to air dry. Remove and discard gloves.
- For disinfection, spray all disassembled parts with a hospital-approved EPA-registered isopropyl alcohol-based disinfectant, such as Cavicide. Let all parts sit visibly wet for at least the contact time indicated in the labeling (2 minutes for Cavicide) before allowing all parts to air dry. See List N: Disinfectants for Use Against COVID-19: https://www.epa.gov/pesticideregistration/list-n-advanced-search-page-disinfectants-coronavirus-covid-19
- Do not use harsh chemicals or abrasives to clean. Do not apply heat. Do not use high concentrations of ammonium in excess of 20% as this may degrade components.
- Store the device at room temperature and relative humidity between 40-60%. Store in a container, if possible, to avoid dust and grease build-up on the device.
www.preptechmed.com// (337)739-5850// [email protected]
References
[xyz-ips snippet=”download-snippet”]