rainbow Acoustic Respiration Sensor for Infants and Neonates User Guide


© 2021 Masimo Corporation
![]() Do Not Re-Use
Do Not Re-Use![]() Not made with natural rubber latex
Not made with natural rubber latex![]() Non-sterile
Non-sterile
Prior to using this sensor, the user should read and understand the Operator’s Manual for the device, monitor and this Directions for Use.
INDICATIONS
The Masimo® Acoustic Respiration Sensor RAS-45 Inf/Neo is indicated for continuous, noninvasive monitoring of respiratory rate (RRa®). The Masimo Acoustic Respiration Sensor RAS-45 Inf/Neo is intended for use with infant and neonatal patients, in hospitals, hospital-type facilities, home environments, and transport within healthcare facilities.The rainbow Acoustic Monitoring® sensors are not indicated for Apnea monitoring.
CONTRAINDICATIONS
The Masimo Acoustic Respiration Sensor RAS-45 Inf/Neo is contraindicated for patients who exhibit allergic reactions to foamrubber products and/or adhesive tape.
DESCRIPTION
The RAS-45 Inf/Neo sensor is for use only with devices containing Masimo rainbow SET® technology.RAS-45 Inf/Neo Sensors are compatible with Masimo rainbow technology V7.14.9.4 and above.
WARNINGS, CAUTIONS AND NOTES
- Always refer to the operator’s manual for the connected monitor, along with these instructions for use for complete instructions.
- When monitoring acoustic respiration (RRa), monitoring oxygenation (functional oxygen saturation of arterial hemoglobin, SpO2) is required.
- All sensors and cables are designed for use with specific monitors. Verify the compatibility of the monitor, cable and sensor before use, otherwise degraded performance and/or patient injury can result.
- Do not use sensors that appear to be damaged or discolored, otherwise degraded performance and/or patient injury can result.
- Do not use sensors during magnetic resonance imaging (MRI) or in an MRI environment.
- Carefully route all patient cables to reduce the risk of patient entanglement or strangulation.
- Do not cover the sensor or apply additional adhesives to the sensor, this may result in inaccurate or no readings.
- Do not place the sensor where it may cover the patient’s nose or mouth.
- Avoid exposing sensors to liquids during use to avoid possible patient harm.
- Do not use tape to secure the sensor to the site; this can cause inaccurate readings. Use of additional tape can cause skin damage, and/or pressure necrosis or damage the sensor.
- Only use the adhesive film provided on the sensor to the secure it to the patient. Excessive pressure can cause skin damage or patient discomfort.
- Check the sensor site (every 8 hours) to ensure skin integrity and to avoid damage or irritation to the skin.
- The site must be checked frequently or per clinical protocol to ensure adequate circulation, skin integrity and correct alignment.
- Periodically check the sensor site for proper adhesion to minimize the risk of inaccurate or no readings.
- Ensure that the placement of the Anchor pad does not tightly stretch the cable to prevent accidental detachment of the sensor due to patient movement.
- The Anchor pad needs to be attached directly to the patient’s skin in order to ensure correct readings.
- Properly apply/place the sensor. Incorrect application or placement may result in inaccurate or no readings.
- Ensure that the cable is not tightly stretched, to prevent accidental detachment of the sensor due to patient movement.
- Avoid placing sensor on patients exposed to environments with excessive noise. This may result in inaccurate or no readings.
- Properly connect the sensor or the oximeter module to the cable to avoid intermittent readings, inaccurate results, or no reading.
- To prevent damage, do not soak or immerse the sensor in any liquid solution. Do not attempt to sterilize the sensor.
- Do not attempt to reuse, reprocess, recondition or recycle Masimo sensors as this may damage the electrical components, potentially leading to patient harm.
- Do not modify or alter the sensor in any way. Alteration or modification may affect performance and/or accuracy.
- If the sensor has been stored in a cold environment, it will need to be brought to room temperature to ensure a correct reading.
- The sensor may not adhere well on extremely diaphoretic patients.
- The RAM® dual cable must be connected to a compatible monitor with Masimo rainbow SET technology with the RRa feature enabled.
- RAS-45 is not for Apnea monitoring or Apnea alarm.
- Caution: Replace the sensor when a replace sensor message is displayed, or when a low SIQ message is consistently displayed after completing the low SIQ troubleshooting steps identified in the monitoring device operator’s manual.
- Note: The sensor is provided with X-Cal® technology to minimize the risk of inaccurate readings and unanticipated loss of patient monitoring. The sensor will provide up to 120 hours of patient monitoring time. After single-patient use, discard sensor.
INSTRUCTIONS
A. Site Selection:Site should be hair-free, cleaned of debris and dry prior to sensor placement. Use an alcohol swab to clean the application site, if needed.
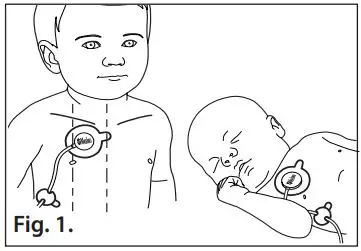
- Refer to Fig. 1. The measuring site is the upper chest. It is recommended that the center of the sensor pad is placed sub clavicular, at the 2nd intercostal towards the right of the patient’s chest.
B. Applying the Sensor:
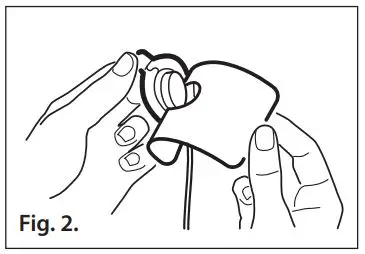
- Refer to Fig. 2. Gently pull the small tab away from the liner to expose the sensor adhesive.

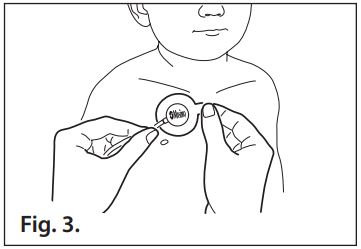
- Refer to Fig. 3. Gently place the sensor onto the application site so that the “Masimo” label is visible.

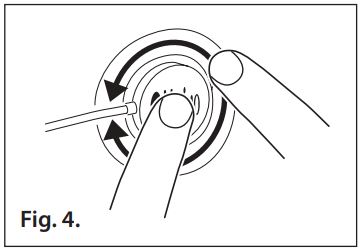
- Refer to Fig. 4. Apply pressure all around the perimeter of the sensor to ensure the adhesive is secure to the patient’s skin. Avoid contact with the exposed sensor adhesive.

- Ensure that the skin of the patient is relaxed and not stretched in any way and that there are no skin folds under the sensor pad.
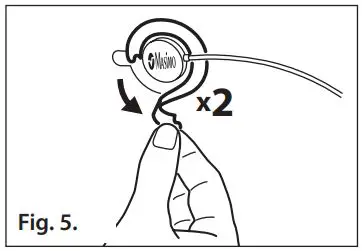
- Refer to Fig. 5. Remove the two piece sensor liner from the top of the sensor.

- Refer to Fig. 6. Remove the anchor liner and attach the anchor pad to the patient’s chest at the level of the armpit.

- Route and secure the sensor cable to reduce the risk of patient entanglement or strangulation.
C. Connecting the RAS-45 Inf/Neo Sensor to the Acoustic Respiration Patient Cable:
- Refer to Fig. 7. Insert the sensor connector into the cable connector, until it locks in place.

- Confirm that the RAS-45 Inf/Neo sensor is properly applied to the patient’s application site and also connected to the patient cable.
- Confirm that the monitor is able to detect a signal (refer to device manual).
Note: If the signals are not detected, select another site on the chest using a new sensor.
D. Disconnecting and Removing the RAS-45 Inf/Neo Sensor: Warning: Disconnect the cable from the sensor prior to removing the sensor from the patient.
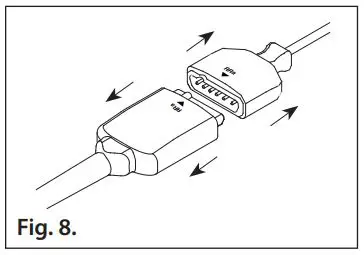
- Refer to Fig. 8. Grasp the cable connector and the sensor connector and pull to disconnect.

- Gently pull on the sensor adhesive tab to stretch and release the sensor from the application site.
- Gently pull on the anchor pad to lift and remove the adhesive from the patient.
Note: When the RAS-45 Inf/Neo sensor is off the patient and is still connected to the system, the sensor may pick up background noise and report a measurement. The sensor should only be connected to the cable while performing patient monitoring. If the patient is not being monitored, the sensor should be disconnected from the cable.
SPECIFICATIONS
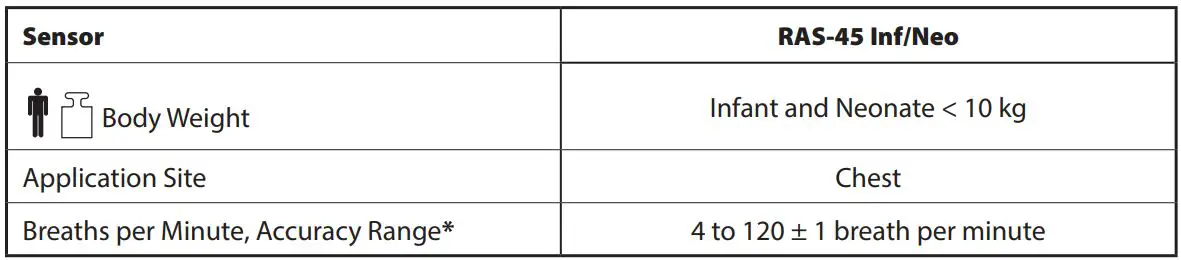
* Respiration rate accuracy for the RAS-45 Info/Neo sensor has been validated for the range of 4 to 120 breaths per minute in bench top testing. Clinical validation was performed with the RAS-45 Info/Neo sensor and monitoring device for up to 82 breaths per minute in infant and neonatal subjects.
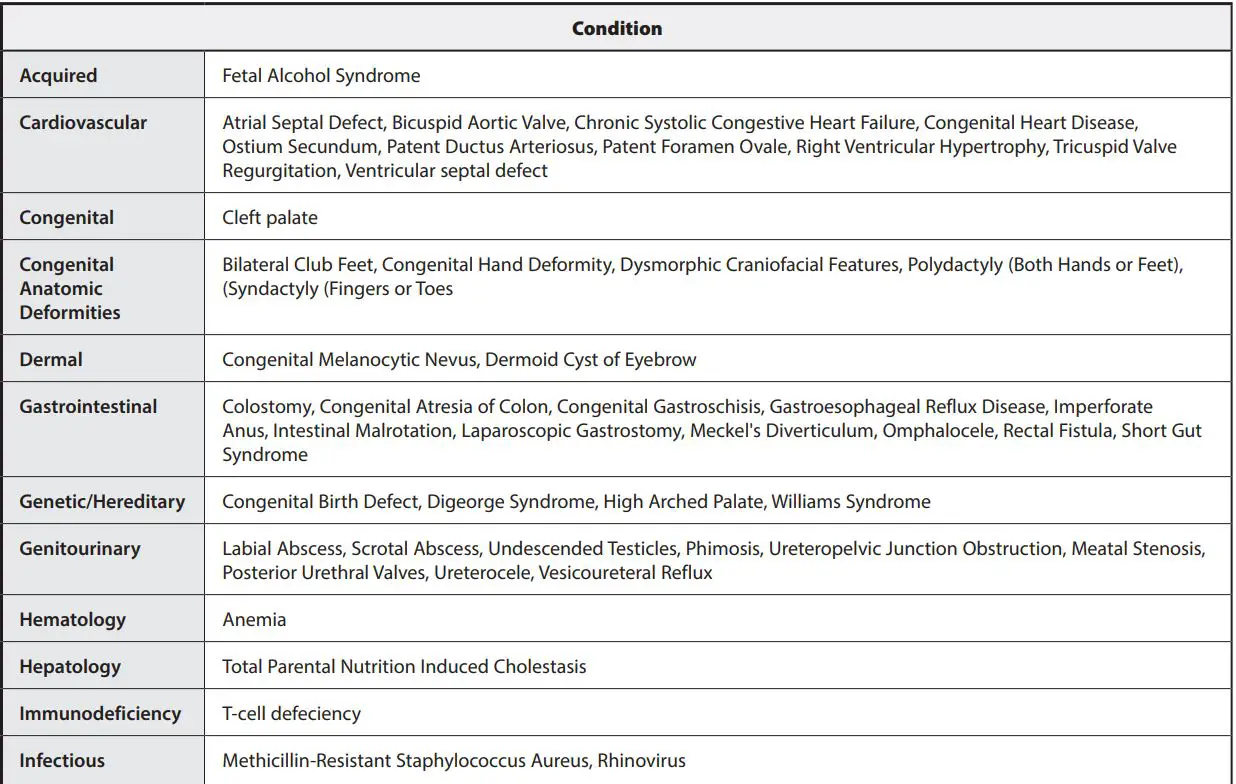
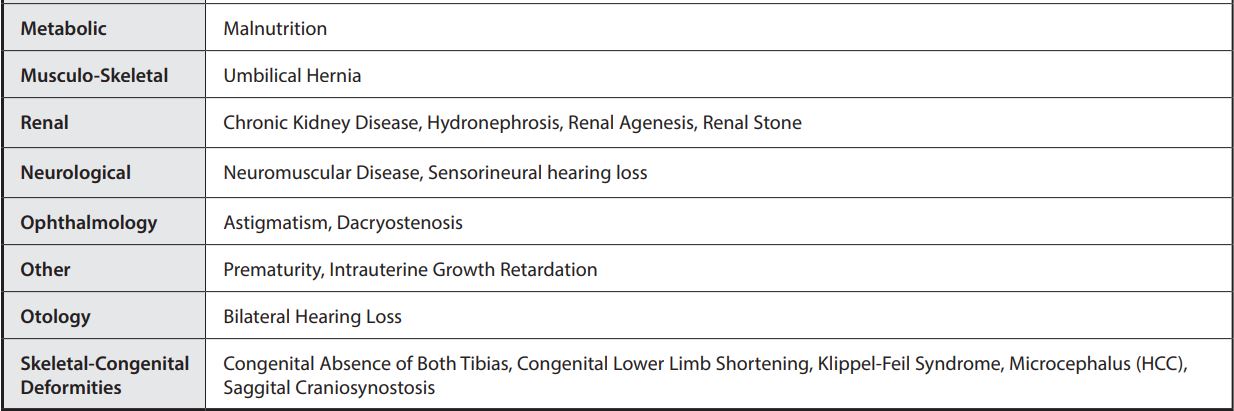
MEDICAL CONDITIONS
Medical Conditions from clinical study of hospitalized patients.
COMPATIBILITY
 This sensor is intended for use only with rainbow Acoustic Monitoring® enabled devices containing Masimo rainbow SET technology or pulse oximetry monitors licensed to use rainbow Acoustic™ compatible sensors.
This sensor is intended for use only with rainbow Acoustic Monitoring® enabled devices containing Masimo rainbow SET technology or pulse oximetry monitors licensed to use rainbow Acoustic™ compatible sensors.



SOFTWARE REQUIREMENT
RAS-45 Inf/Neo Sensors are compatible with Masimo rainbow technology V7.14.9.4 and above.
WARRANTY
Masimo warrants to the initial buyer only that these products, when used in accordance with the directions provided with the Products by Masimo, will be free of defects in materials and workmanship for a period of six (6) months. Single use products are warranted for single patient use only.
THE FOREGOING IS THE SOLE AND EXCLUSIVE WARRANTY APPLICABLE TO THE PRODUCTS SOLD BY MASIMO TO BUYER. MASIMO EXPRESSLY DISCLAIMS ALL OTHER ORAL, EXPRESS OR IMPLIED WARRANTIES, INCLUDING WITHOUT LIMITATION ANY WARRANTIES OF MERCHANTABILITY OR FITNESS FOR PARTICULAR PURPOSE. MASIMO’S SOLE OBLIGATION AND BUYER’S EXCLUSIVE REMEDY FOR BREACH OF ANY WARRANTY SHALL BE, AT MASIMO’S OPTION, TO REPAIR OR REPLACE THE PRODUCT.
WARRANTY EXCLUSIONS
This warranty does not extend to any product that has been used in violation of the operating instructions supplied with the product, or has been subject to misuse, neglect, accident or externally created damage. This warranty does not extend to any product that has been connected to any unintended device or system, has been modified, or has been disassembled or reassembled. This warranty does not extend to sensors or patient cables that have been reprocessed, reconditioned or recycled.
IN NO EVENT SHALL MASIMO BE LIABLE TO BUYER OR ANY OTHER PERSON FOR ANY INCIDENTAL, INDIRECT, SPECIAL OR CONSEQUENTIAL DAMAGES (INCLUDING WITHOUT LIMITATION LOST PROFITS), EVEN IF ADVISED OF THE POSSIBILITY THEREOF. IN NO EVENT SHALL MASIMO’S LIABILITY ARISING FROM ANY PRODUCTS SOLD TO BUYER (UNDER A CONTRACT, WARRANTY, TORT OR OTHER CLAIM) EXCEED THE AMOUNT PAID BY BUYER FOR THE LOT OF PRODUCT(S) INVOLVED IN SUCH CLAIM. IN NO EVENT SHALL MASIMO BE LIABLE FOR ANY DAMAGES ASSOCIATED WITH A PRODUCT THAT HAS BEEN REPROCESSED, RECONDITIONED OR RECYCLED. THE LIMITATIONS IN THIS SECTION SHALL NOT BE DEEMED TO PRECLUDE ANY LIABILITY THAT, UNDER APPLICABLE PRODUCTS LIABILITY LAW, CANNOT LEGALLY BE PRECLUDED BY CONTRACT.
NO IMPLIED LICENSETHIS SINGLE-PATIENT SENSOR IS LICENSED TO YOU UNDER THE PATENTS OWNED BY MASIMO FOR SINGLE-PATIENT USE ONLY. BY ACCEPTANCE OR USE OF THIS PRODUCT, YOU ACKNOWLEDGE AND AGREE THAT NO LICENSE IS GRANTED FOR USE OF THIS PRODUCT WITH MORE THAN A SINGLE PATIENT.AFTER SINGLE-PATIENT USE, DISCARD SENSOR. PURCHASE OR POSSESSION OF THIS SENSOR CONFERS NO EXPRESS OR IMPLIED LICENSE TO USE THE SENSOR WITH ANY DEVICE WHICH IS NOT SEPARATELY AUTHORIZED TO USE RD SENSORS.
CAUTION: FEDERAL LAW (U.S.A.) RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. For professional use. See instructions for use for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.If you encounter any serious incident with product, please notify the competent authority in your country and the manufacturer.The following symbols may appear on the product or product labeling:

http://www.Masimo.com/TechDocs
Patents: http://www.masimo.com/patents.htm Masimo, , rainbow SET, rainbow Acoustic, rainbow, rainbow Acoustic Monitoring, RAM, X-Cal, RRa, and SET are federally registered trademarks of Masimo Corporation.
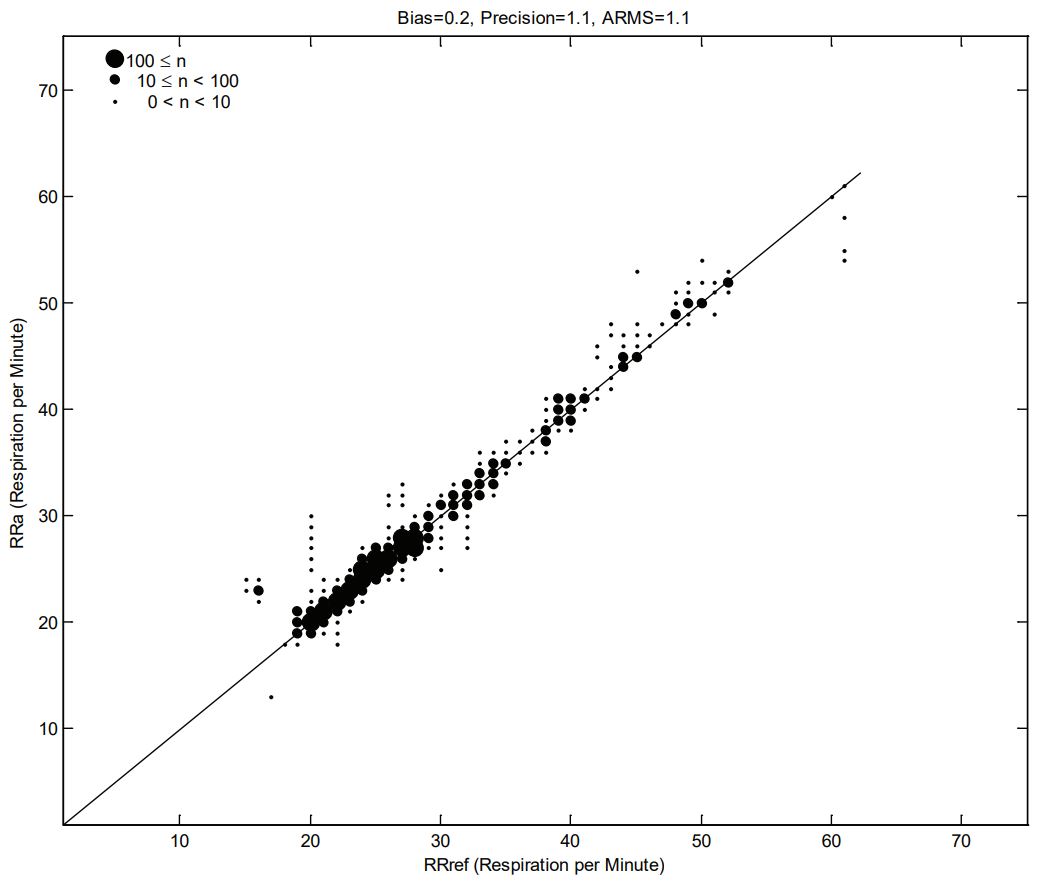
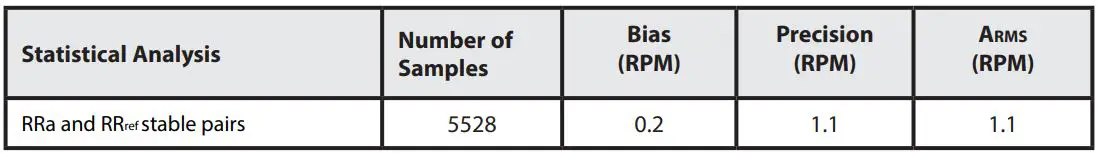
RESPIRATION RATE ACCURACY
The following plots showing correlation between RAS-45 Inf/Neo Sensor (RRa) and RRref stable respiration rate estimation RRref:






![]()
![]()
EU Authorized Representative for Masimo Corporation:(EC|REP)MDSS GmbHSchiff graben 41D-30175 Hannover, Germany
![]()
![]()
10075G-eIFU-0321
References
[xyz-ips snippet=”download-snippet”]