Bioadvanced Science-based Solutions 3-in-1 Insect, Disease & Mite Control Ready-to-spray Instruction Guide

SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING

SECTION 2: HAZARDS IDENTIFICATION
Classification in accordance with regulation HCS 29CFR §1910.1200Reproductive toxicity : Category 2

Signal word: Warning
Hazard statementsSuspected of damaging fertility or the unborn child.
Precautionary statementsObtain special instructions before use.Do not handle until all safety precautions have been read and understood.Wear protective gloves/protective clothing/eye protection/face protection.IF exposed or concerned: Get medical advice/attention.Store locked up.Dispose of contents/container in accordance with local regulation.
Other hazardsNo other hazards known.
SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS
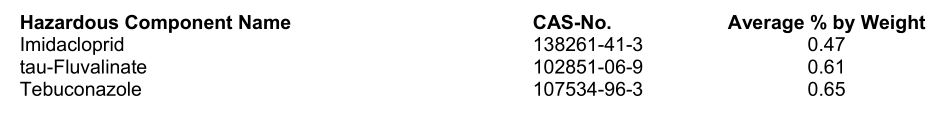
SECTION 4: FIRST AID MEASURES
Description of first aid measures
General adviceWhen possible, have the product container or label with you when calling a poison control center or doctor or going for treatment.
InhalationMove to fresh air. If person is not breathing, call 911 or an ambulance, then give artificial respiration, preferably mouth-to-mouth if possible. Call a physician or poison control center immediately.
Skin contactTake off contaminated clothing and shoes immediately. Wash off immediately with plenty of water for at least 15 minutes. Call a physician or poison control center immediately.
Eye contactHold eye open and rinse slowly and gently with water for 15-20 minutes. Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eye. Call a physician or poison control center immediately.
IngestionCall a physician or poison control center immediately. Rinse out mouth and give water in small sips to drink. DO NOT induce vomiting unless directed to do so by a physician or poison control center. Never give anything by mouth to an unconscious person. Do not leave victim unattended.
Most important symptoms and effects, both acute and delayed SymptomsTo date no symptoms are known.
Indication of any immediate medical attention and special treatment needed TreatmentAppropriate supportive and symptomatic treatment as indicated by the patient’s condition is recommended. There is no specific antidote
SECTION 5: FIREFIGHTING MEASURES

SECTION 6: ACCIDENTAL RELEASE MEASURES

SECTION 7: HANDLING AND STORAGE

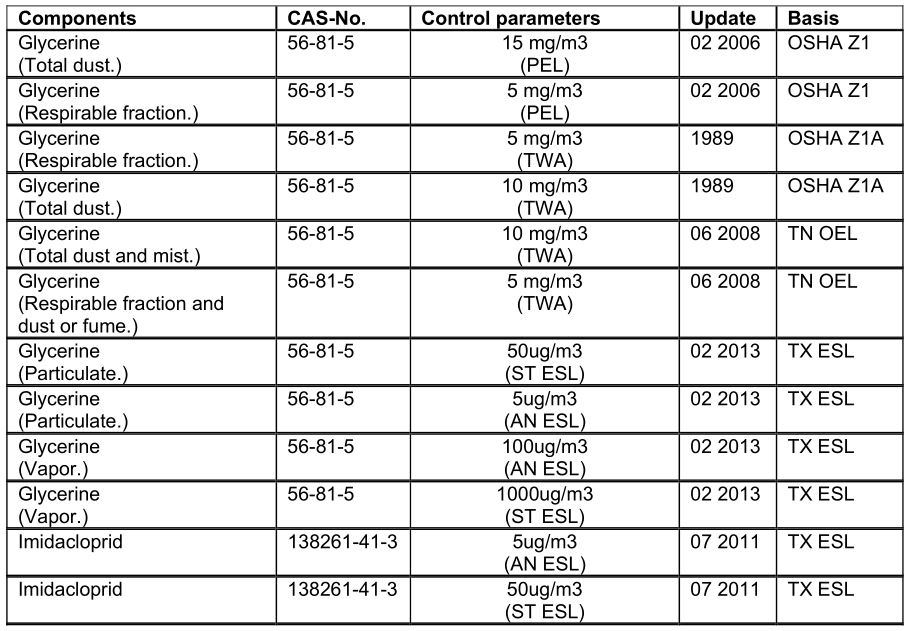
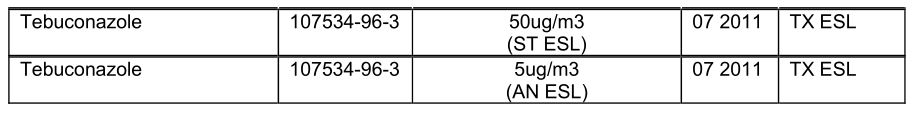
SECTION 8: EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Exposure controls

SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
- Appearance white to light beige
- Physical State Liquid
- Odor almost odourless
- Odour Threshold no data available
- pH >= 3 at 100 %
- Vapor Pressure no data available
- Vapor Density (Air = 1) no data available
- Density ca. 1.03 g/cm³ at 20 °C
- Evapouration rate no data available
- Boiling Point no data available
- Melting / Freezing Point no data available
- Water solubility dispersible
- Minimum Ignition Energy not applicable
- Decomposition temperature no data available
- Partition coefficient: n-octanol/water no data available
- Viscosity 100 – 500 mPa.s
- Flash point > 93.4 °C
- Autoignition temperature no data available
- Lower explosion limit no data available
- Upper explosion limit no data available
- Explosivity not applicable
SECTION 10: STABILITY AND REACTIVITY
- Reactivity Thermal decompositionno data available
- Chemical stabilityStable under normal conditions.
- Possibility of hazardous reactionsNo dangerous reaction known under conditions of normal use.
- Conditions to avoidno data available
- Incompatible materialsno data available
- Hazardous decomposition productsNo decomposition products expected under normal conditions of use.
SECTION 11: TOXICOLOGICAL INFORMATION
- Exposure routesInhalation, Skin Absorption, Eye contact, Ingestion
- Immediate Effects EyeMay cause mild irritation to eyes.
- SkinHarmful if absorbed through skin. May cause slight irritation.
- IngestionHarmful if swallowed.
- InhalationHarmful if inhaled.
- Information on toxicological effects
- Acute oral toxicityLD50 (female rat) > 2,000 mg/kg
- Acute inhalation toxicityLC50 (male/female combined rat) > 5.1 mg/lExposure time: 4 hDetermined in the form of a respirable aerosol.(actual)LC50 (male/female combined rat) > 20.4 mg/lExposure time: 1 hDetermined in the form of a respirable aerosol.Extrapolated from the 4 hr LC50.(actual)
- Acute dermal toxicityLD50 (male/female combined rat) > 4,000 mg/kg
- Skin irritationSlight irritation (rabbit)
- Eye irritationMinimally irritating. (rabbit)
- SensitisationNon-sensitizing. (guinea pig)
Assessment repeated dose toxicityImidacloprid did not cause specific target organ toxicity in experimental animal studies.Tau-fluvalinate did not cause specific target organ toxicity in experimental animal studies.Tebuconazole did not cause specific target organ toxicity in experimental animal studies.
Assessment MutagenicityImidacloprid was not mutagenic or genotoxic based on the overall weight of evidence in a battery of in vitro and in vivo tests.Tau-fluvalinate was not mutagenic or genotoxic in a battery of in vitro and in vivo tests.Tebuconazole was not mutagenic or genotoxic in a battery of in vitro and in vivo tests.
Assessment CarcinogenicityImidacloprid was not carcinogenic in lifetime feeding studies in rats and mice.Tau-fluvalinate was not carcinogenic in lifetime feeding studies in rats and mice.Tebuconazole caused at high dose levels an increased incidence of tumours in mice in the following organ(s): liver. The mechanism of tumour formation is not considered to be relevant to man.
ACGIHNone.
NTPNone.
IARCNone.
OSHANone.
Assessment toxicity to reproductionImidacloprid caused reproduction toxicity in a two-generation study in rats only at dose levels also toxic to the parent animals. The reproduction toxicity seen with Imidacloprid is related to parental toxicity. Tau-fluvalinate did not cause reproductive toxicity in a two-generation study in rats. Tebuconazole caused reproduction toxicity in a two-generation study in rats only at dose levels also toxic to the parent animals. The reproduction toxicity seen with Tebuconazole is related to parental toxicity.
Assessment developmental toxicityImidacloprid caused developmental toxicity only at dose levels toxic to the dams. The developmental effects seen with Imidacloprid are related to maternal toxicity. Tau-fluvalinate did not cause developmental toxicity in rats and rabbits. Tebuconazole caused developmental toxicity only at dose levels toxic to the dams. Tebuconazole caused an increased incidence of post implantation losses, an increased incidence of non-specific malformations.
Further informationOnly acute toxicity studies have been performed on the formulated product.The non-acute information pertains to the active ingredient(s).
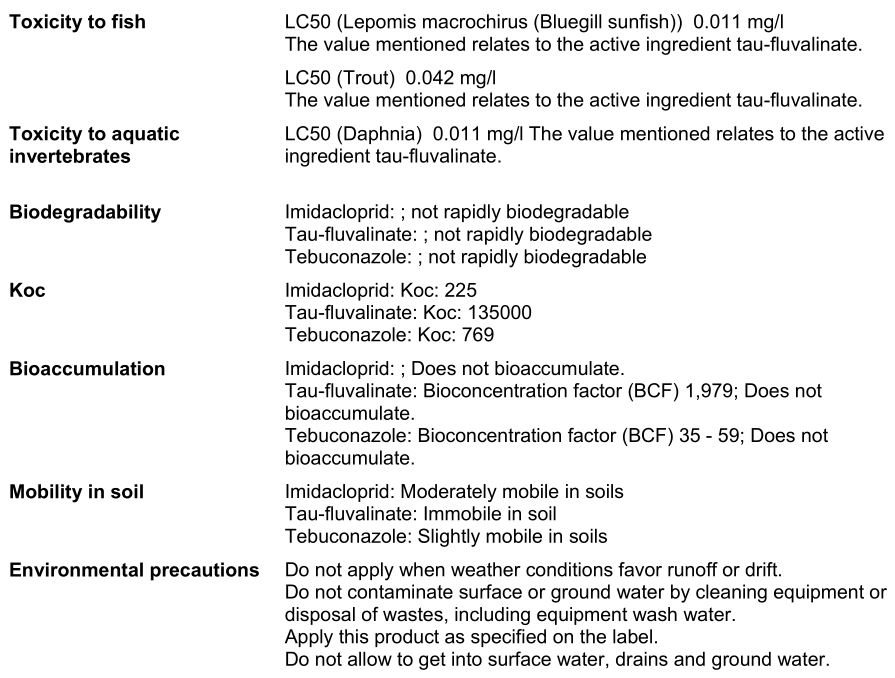
SECTION 12: ECOLOGICAL INFORMATION

SECTION 13: DISPOSAL CONSIDERATIONS
Waste treatment methods ProductIt is best to use all of the product in accordance with label directions. If it is necessary to dispose of unused product, please follow container label instructions and applicable local guidelines. Never place unused product down any indoor or outdoor drain.
Contaminated packagingDo not re-use empty containers. Place empty container in trash.
RCRA InformationCharacterization and proper disposal of this material as a special or hazardous waste is dependent upon Federal, State and local laws and are the user’s responsibility. RCRA classification may apply.
SECTION 14: TRANSPORT INFORMATION

SECTION 15: REGULATORY INFORMATION

SECTION 16: OTHER INFORMATION

Bioadvanced Science-based Solutions 3-in-1 Insect, Disease & Mite Control Ready-to-spray Instruction Guide – Bioadvanced Science-based Solutions 3-in-1 Insect, Disease & Mite Control Ready-to-spray Instruction Guide –
[xyz-ips snippet=”download-snippet”]

