Instructions for Healthcare Providers and Facilities: SCONETM Device
The U.S. Food and Drug Administration has issued an Emergency Use Authorization (EUA) for SCONETM, for use by a healthcare provider (HCP) as an extra layer of barrier protection in addition to personal protective equipment (PPE) to prevent HCP exposure to pathogenic biological airborne particulates by providing isolation of hospitalized patients with suspected or confirmed diagnosis of COVID-19, at the time of definitive airway management, or when performing airway-related medical procedures, or during certain transport of such patients during the COVID-19 pandemic.Authorized non-transport use of SCONETM is only for definitive airway management (e.g., intubation, extubation and suctioning airways), when performing any airway-related medical procedures (e.g., high flow nasal cannula oxygen treatments, nebulizer treatments, manipulation of an oxygen mask or continuous positive airway pressure/bilevel positive airway pressure [CPAP/BiPAP] mask use, airway suctioning, percussion and postural drainage), or during certain patient transport. Authorized use of SCONETM during patient transport is only within a hospital setting for temporary transfer with direct admission within the hospital in the presence of a registered nurse or physician. The patient should have constant monitoring of vital signs, electrocardiogram (ECG/EKG), SpO2% (oxygen saturation), End-tidal carbon dioxide (EtCO2) if available throughout transport. For all authorized uses, the patient should always have supplemental oxygen during use of SCONETM. SCONETM can be used longer than 30 minutes only if constant monitoring of vital signs is maintained and only for as long as required to complete the indicated procedures. If EtCO2 monitoring is not possible, then the use of the SCONE is limited to a maximum of 30 minutes with negative pressure suction on and under direct observation, while ECG/EKG and SpO2% must be monitored at all times.
SCONE™ has not been FDA-approved or cleared for this use; SCONE has been authorized for emergency use by FDA under a EUA. SCONETM has been authorized only for the duration of the COVID-19 public health emergency declaration that circumstances exist justifying the authorization of the emergency use of medical devices under section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
HCP should follow these instructions, as well as procedures at their healthcare facility to use SCONETM.
The instructions below are to assist in using SCONETM. SCONETM is an adjunctive protective barrier designed to mitigate risk to HCP. SCONETM is not meant to be a standalone unit of PPE. SCONETM should always be used with appropriate PPE and pursuant to the guidance of your institution.
All connections should be tightly secured and checked frequently. Any time anyone is within SCONETM, direct observation is required. Inspect SCONETM prior to use. Any wear/tear of the chamber or other signs of degradation on SCONETM must promptly be reported to SCONETM Medical Solutions Inc. The healthcare facility must not use on patients and must dispose of such a SCONETM. Rx Only WARNINGS:
WARNINGS:
- The flammability of SCONETM has not been tested. No interventions that could create a spark or be a flammable source should be used within SCONETM.
- Remove SCONETM and use the standard of care if it impedes the ability to perform the standard of care, or if there is difficulty visualizing or identifying anatomic landmarks, or if it impedes the ability to intubate the patient after the first try.
- Prolonged use of SCONETM may induce hypercarbia in a spontaneously breathing patient. SCONETM should be used with medical airflow and suction both on and working, under direct observation, and with EtCO2 monitoring if available.
- Patient transport must only occur within a hospital setting for temporary transfer with direct admission within the hospital in the presence of a registered nurse or physician. Maintenance of negative pressure with adequate airflow must be assured. All patients should be on supplemental oxygen. Patients must have continuous monitoring of SpO2%, vital signs, ECG/EKG, and EtCO2 if available during transport.
- Use caution prior to use on non-sedated or lightly sedated patients with severe claustrophobia and/or confined space anxiety.
- When using SCONETM, patients should always be receiving supplemental oxygen.
- Patients with diminished hearing may have difficulty understanding the provider while inside SCONETM. · SCONETM is a single-use device and should be disposed of following the disposal instructions after use. · If SCONETM is accidentally folded or the air-ports are blocked, this may result in patient injury · Delayed emergency removal of the device may result in patient injury
CONTRAINDICATIONS:
SCONETM is not authorized for use on:
- Patients needing emergent endotracheal intubation with severe hypoxemia
- Patients with an anticipated or known history of difficult airway
- Patients with other anatomical abnormalities that might interfere with clinical care including decreased neck mobility from arthritis or other causes
- Patients with communication disorders that might interfere with clinical care
- Children under 45 lbs.
- Patients with an anticipated or known history of claustrophobia
- Patients with communication disorders that might interfere with clinical care
- Bariatric patients
- Patients with uncontrolled movements that may prevent the patient from being able to remain enclosed in the tent enclosure
- Patients in elderly care centers (non-hospital environment)
- Patients in ambulance transport
SCONE™ Instructions for Use
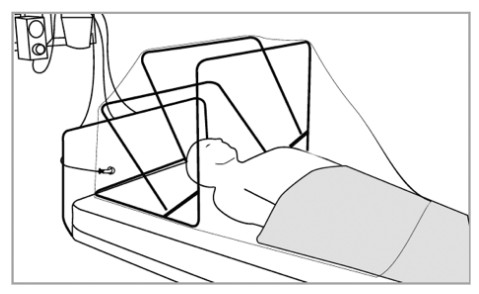
Device Description:SCONE™ is a single-use, clear, negative pressure chamber that attaches to a standard hospital or surgical bed or stretchers and extends around the patient’s head, neck, and shoulders. Access holes, sealed by vinyl access covers, are built into the chamber to allow for isolated patient access. The negative pressure environment is generated via wall-mounted hospital vacuum lines or 1-2 negative pressure pumps equipped with in-line high-efficiency particulate air (HEPA) filter(s). The SCONE™ unit acts as an added layer of a physical barrier in addition to PPE to prevent HCP exposure to pathogenic biological airborne particulates. SCONE™ will be available to HCP and healthcare facilities.
Items You Will Need:
- SCONE™ Device
- 1 or 2 Facility Wall-Mounted Pressure Regulators (capable of maintaining continuous high suction at 200mmHg to produce 30 LPM per line) OR Portable Vacuum Pump(s) (capable of maintaining continuous high suction at 200mmHg to produce 30 LPM per line) with Inline HEPA Filter(s) (single use only; 0.3 µm or better filtration)
- 1 Facility Wall-Mounted Oxygen Delivery Regulator
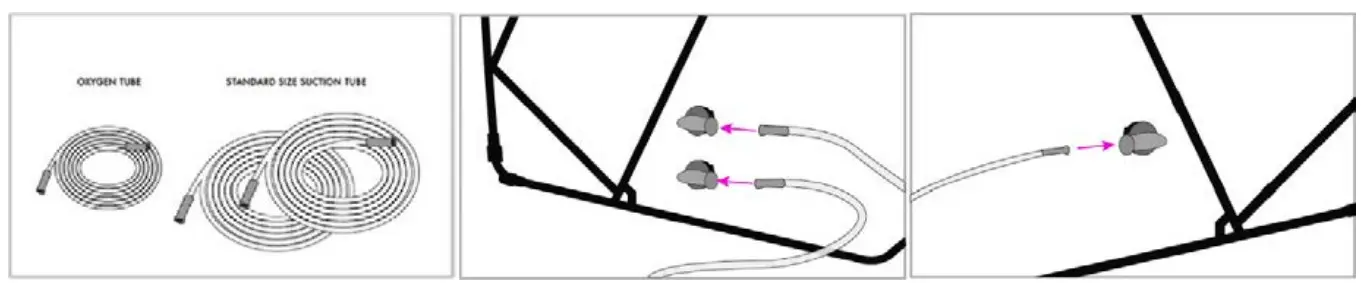
- 1 or 2 Healthcare Facility Standard Suction Hose Lines (minimum 1/4″ID) (Single Use Only)
- 1 Healthcare Facility Standard Oxygen Line (usually 3/16″ID) (Single Use Only)
- 1 Blanket for Patient
- Endotracheal tube
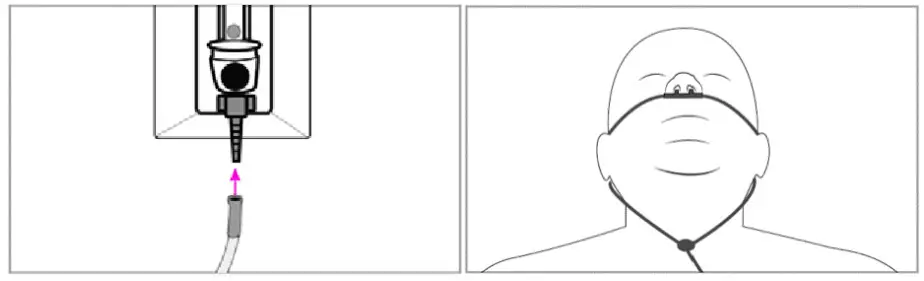
- Nasal cannula
- Oxygen mask
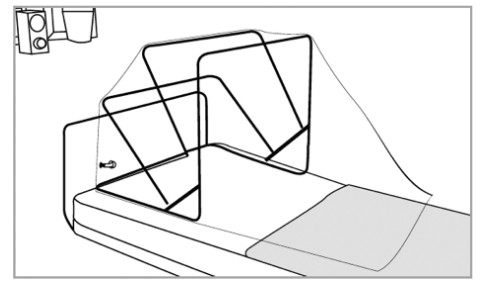
Device Components:

Setup Instructions:
- PPE IS REQUIRED AT ALL TIMES WHEN OPERATING SCONE™.
- Remove SCONE™ from packaging. If desired, keep the shipping box for safer disposable.

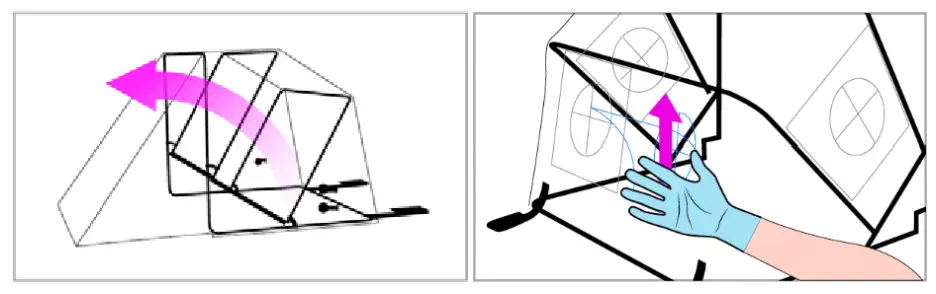
- Unfold SCONE™ chamber by fully extending the frame and locking the hinges on each side. Verify that the 4 arm access hole covers are properly sealed.

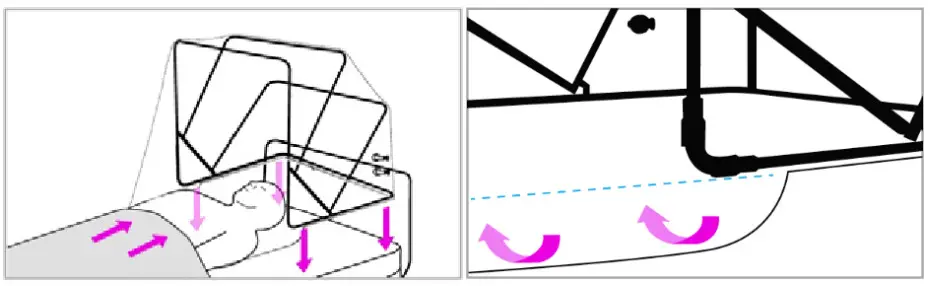
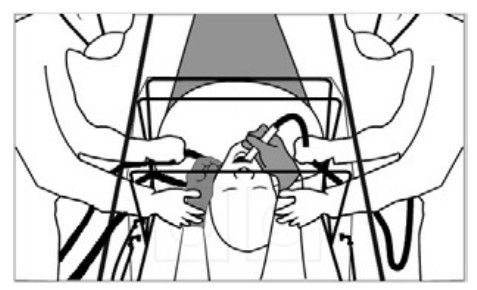
- Place SCONE™ at the head of the bed over the patient’s upper torso (head, neck, and shoulders) and tuck the slide flaps underneath the bottom front part of the frame. Drape a blanket across the patient over the front edge of SCONE™ overlay to create a proper seal.

- Attach SCONE™ to the bed using the hook and loop fastener straps located at the back of the device. These may be attached and reattached as needed depending on whether the patient is lying supine or elevated.

- Locate the suction and oxygen port adapters sealed by caps on either side of the device. The side with 2 port adapters is for suction tubing and the side with 1 port adapter is for oxygen tubing. The port adapter caps will hang off and remain attached to the adapter when in use. If an adapter port is not in use, the cap should always be sealed.

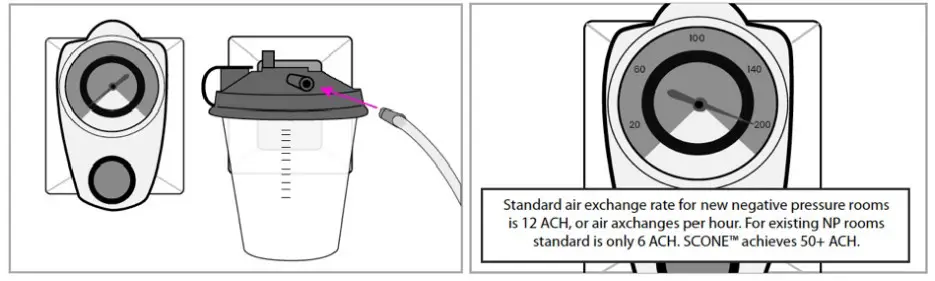
- Connect 1 or 2 standard healthcare facility wall suction hoses (minimum 1/4″ID) to the wall-mounted pressure gauge or portable vacuum pump(s) w/ inline HEPA filter(s) and set to continuous high suction (200mmHg) to achieve a minimum of 50-70 LPM of airflow (30 LPM per line) for a minimum clearance time of 5 minutes. When using one or two portable pump(s), each line should be connected to a single portable pump. Note, the tubing and HEPA filter(s) should be single-use only. If only one line is available, set the pressure to continuous high suction (200mmHg) but allow a minimum of 10 minutes of clearance time to ensure full evacuation of airborne particulates.

- Connect 1 standard healthcare facility oxygen line to SCONE™ port adapter, which is a single port located on the opposite side of the 2 port adapters (suction adapters) on the outside of SCONE™. Connect the other end of the oxygen line to wall regulator mount and set to 10 LPM.Note, if a nasal cannula will be in use for the patient, skip this step and do not connect an oxygen line to the Scone device. Instead, run the nasal cannula through the draping to the patient. Ensure the single port adapter cap is over the port.

- Monitor patient vitals during use. (EKG, SpO2, EtCO2, etc.)
- SCONE™ is intended for use during airway management and aerosol-generating procedures with continuous suction or for transport of patients in healthcare facilities for a maximum duration of 30 minutes. Patient vitals should be continuously monitored during use. SCONE ™may be used longer than 30 minutes only if constant monitoring of vital signs is maintained and only for as long as required to complete the indicated procedures. If End-Tidal CO2 monitoring is not possible, then use of the SCONE is limited to a maximum of 30 minutes with negative pressure suction on and under direct observation, while ECG/EKG and SpO2% must be monitored at all times.
Performing a Procedure while using SCONE™
- PPE IS REQUIRED AT ALL TIMES WHEN OPERATING SCONE™.
- Instruments (e.g. EKG, SpO2, EtCO2, etc.) for monitoring may be fed up under the front draping next to the patient’s torso, making sure to keep an appropriate seal around the instrumentation. Procedural instrumentation may be passed through arm access holes or also fed up under the front draping.

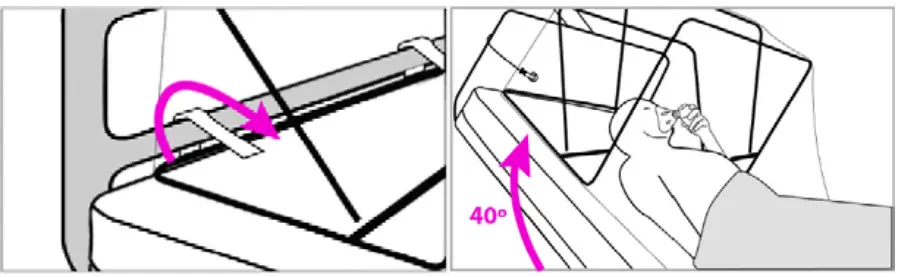
- Open arm access flaps as needed to access the patient during the procedure. Keep them sealed when not in use.

- After the procedure, wait for a minimum of 5 (dual suction) or 10 (single-suction) minutes for SCONE™ to clear particulates before removing.

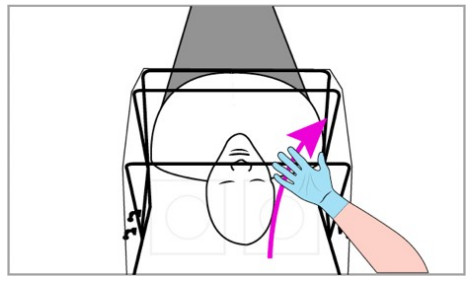
Emergency Removal Instructions
- PPE IS REQUIRED AT ALL TIMES WHEN OPERATING SCONE™.
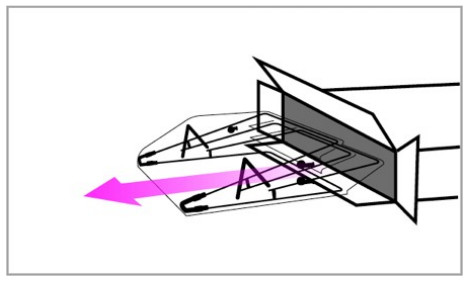
- Detach the hook and loop fastener straps from the bed and pull SCONE™ off the patient. Set aside.
Disposal Instructions
- PPE IS REQUIRED AT ALL TIMES WHEN DISPOSING OF SCONE™.
- Once the patient has been removed from SCONE™, wait for the device to clear the particulates in the environment (minimum of 5 or 10 minutes depending on configuration).

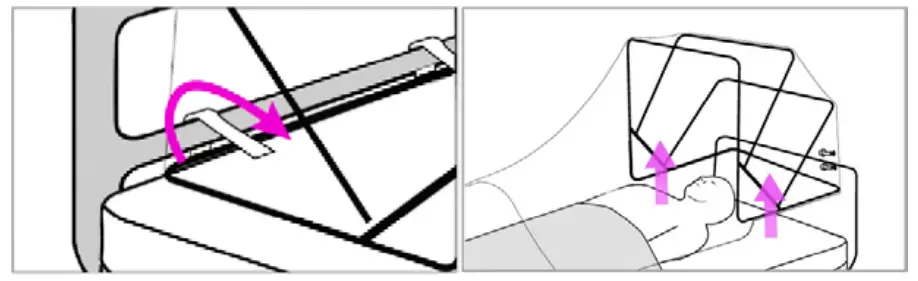
- Unlock hinges and fold SCONE™ back down into collapse position, careful to not expose the underneath part of the overlay. Place SCONE™ back into the shipping box (if available).

- Follow the facility’s policy and protocol for disposal of biohazardous waste.

- SCONE™ is a single-patient-use item and is not to be reused under any circumstances.
 SCONE™ is a one-time use device. The device after use is a biohazard and requires proper disposal per facility standard procedures. Please instruct all personnel involved in the breakdown and disposal of SCONE™ to be dressed in full PPE.
SCONE™ is a one-time use device. The device after use is a biohazard and requires proper disposal per facility standard procedures. Please instruct all personnel involved in the breakdown and disposal of SCONE™ to be dressed in full PPE.
[xyz-ips snippet=”download-snippet”]