COVID-19 Ag
STANDARDTM Q COVID-19 Ag Test
PLEASE READ CAREFULLY BEFORE YOU PERFORM THE TEST.
KIT CONTENTS
 Test device (individually in a
Test device (individually in a
foil pouch with desiccant).

Extraction buffer tube.

Nozzle cap
![]()
Sterileswab

Instructions for use
PREPARATION

1.Carefully read instructions for using theSTANDARD Q COVID-19 Ag Test.

2.Check the expiry date at the back of the foil pouch. Do not use the kit, if expiry date has passed.

3.Check the test device and the desiccant pack in the foil pouch.
<Foil pouch>

<Test device>

![]() Yellow: ValidGreen: Invalid
Yellow: ValidGreen: Invalid
<Desiccant>
COLLECTION OF SPECIMEN
[Nasopharyngeal swab]
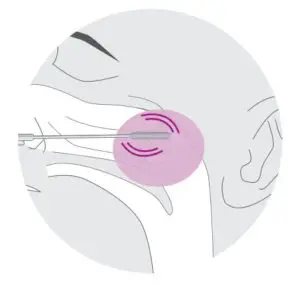
1.Insert a sterile swab into the nostril of the patient, swab over the surface of theposterior nasopharynx. Withdraw the sterile swab from the nasal cavity.

2.Insert the swab into an extraction buffer tube. While squeezing the buff er tube, stir the swab more than 5 times.

3.Remove the swab while squeezing thesides of the tube to extract the liquid fromthe swab.
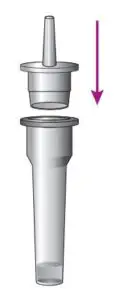
4.Press the nozzle cap tightly onto the tube.
[Specimens in transport media]

1.Using a micropipette, collect the 350µl of specimen from the collection cup or VTM. Mix the specimen with an extraction buffer.

2.Press the nozzle cap tightly onto the tube.
ANALYSIS OF SPECIMEN

1.Apply 3 drops of extracted specimen to the specimen well of the test device.
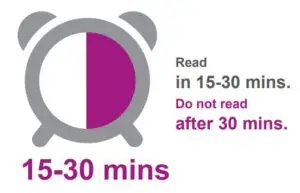
2.Read the test result in 15-30 minutes.
INTERPRETATION OF TEST RESULT

- A colored band will appear in the top section of the result window to show that the test is working properly. This band is control line (C).
- A colored band will appear in the lower section of the result window. This band is test line of SARS-CoV-2 antigen (T).
- Even if the control line is faint, or the test line isn’t uniform, the test should be considered to be performed properly and the test result should be interpreted as a positive result.
- The presence of any line no matter how faint the result is considered positive.
- Positive results should be considered in conjunction with the clinical history and other data available.
EXPLANATION AND SUMMARY
[Introduction]
Coronavirus is a single-stranded positive-sense RNA virus with an envelope of about 80 to 120 nm in diameter. Its genetic material is the largest of all RNA viruses and is an important pathogen of many domestic animals, pets, and human diseases. It can cause a variety of acute and chronic diseases. Common signs of a person infectedwith a coronavirus include respiratory symptoms, fever, cough, shortness of breath, and dyspnea. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death. The 2019 new coronavirus, or “SARS-CoV-2 (COVID-19)”, was discovered because of Wuhan Viral Pneumonia cases in 2019, and was named by the World Health Organization on January 12, 2020, confirming that it can causecolds and the Middle East Respiratory Syndrome (MERS) and more serious diseases such as acute respiratory syndrome (SARS). This kit is helpful for the auxiliary diagnosis of coronavirus infection. The test results are for clinical reference only and cannot be used as a basis for confirming or excluding cases alone.
[Intended use]
STANDARD Q COVID-19 Ag Test is a rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 present in human nasopharynx. This test is for administration by healthcare workers and labs only, as an aid to early diagnosis of SARS-CoV-2 infection in patient with clinical symptoms with SARS-CoV-2 infection. It provides only an initial screening test result. This product is strictly for medical professional use only and not intended for personal use. The administration of the test and the interpretation of the results shouldbe done by a trained health professional. The result of this test should not be the sole basis for the diagnosis; confirmatory testing is required.
[Test principle]
STANDARD Q COVID-19 Ag Test has two pre-coated lines, “C” Control line, “T” Test line on the surface of the nitrocellulose membrane. Both the control line and test line in the result window are not visible before applying any specimens. Mouse monoclonal anti-SARS-CoV-2 antibody is coated on the test line region and mouse monoclonal anti-Chicken IgY antibody is coated on the control line region. Mouse monoclonal anti-SARS-CoV-2antibody conjugated with color particles are used as detectors for SARS-CoV-2 antigen device. During the test, SARS-CoV-2 antigen in the specimen interact with monoclonal anti-SARS-CoV-2 antibody conjugated with color particles making antigen-antibody color particle complex. This complex migrates on the membrane via capillary action until the test line, where it will be captured by the mouse monoclonal anti-SARS-CoV-2 antibody. A coloredtest line would be visible in the result window if SARS-CoV-2 antigens are present in the specimen. The intensity of colored test line will vary depending upon the amount of SARS-CoV-2 antigen present in the specimen. If SARSCoV-2 antigens are not present in the specimen, then no color appears in the test line. The control line is used for procedural control, and should always appear if the test procedure is performed properly and the test reagents of the control line are working.
[Kit contents]
- Test device (individually in a foil pouch with desiccant)
- Extraction buffer tube
- Nozzle cap
- Sterile swab
- Instructions for use
KIT STORAGE AND STABILITY
Store the kit at 2-30°C / 36-86°F out of direct sunlight. Kit materials are stable until the expiration date printed on the outer box. Do not freeze the kit.
WARNINGS AND PRECAUTIONS
- Do not re-use the test kit.
- Do not use the test kit if the pouch is damaged or the seal is broken.
- Do not use the extraction buffer tube of another lot.
- Do not smoke, drink or eat while handling specimen.
- Wear personal protective equipment, such as gloves and lab coats when handling kit reagents. Wash hands thoroughly after the tests are done.
- Clean up spills thoroughly using an appropriate disinfectant.
- Handle all specimens as if they contain infectious agents.
- Observe established precautions against microbiological hazards throughout testing procedures.
- Dispose of all specimens and materials used to perform the test as bio-hazard waste. Laboratory chemical and biohazard wastes must be handled and discarded in accordance with all local, state, and national regulations.
- Desiccant in foil pouch is to absorb moisture and keep humidity from affecting products. If the moistureindicating desiccant beads change from yellow to green, the test device in the pouch should be discarded.
SPECIMEN COLLECTION AND PREPARATION
- To collect a nasopharyngeal swab specimen, insert a sterile swab into the nostril of the patient, reaching thesurface of the posterior nasopharynx.
- Using gentle rotation, push the swab until resistance is met at the level of the turbinate.
- Rotate the swab a few times against the nasopharyngeal wall.
- Remove the swab from the nostril carefully.
- Specimen should be tested as soon as possible after collection.
- Specimens may be stored at room temperature for up to 1 hours or at 2-8°C/ 36-46°F for up to 4 hours prior to testing.
![]() If the specimen storage condition is out of instructions as below, do not use.
If the specimen storage condition is out of instructions as below, do not use.
- The Nasopharyngeal swab is stored in extraction buffer for more than 4 hours at 5±3℃ or 1 hour at 20±5℃.
- Freezing and thawing of Nasopharyngeal swab or the specimen in UTM is more than 1 cycle or 3 cycles.
- The Nasopharyngeal swab is stored in UTM for more than 12 hours at 5±3℃ or 8 hours at 20±5℃.
[Transport medium]


When using viral transport medium (VTM), it is important to ensure that the VTM containing the sample is warmed to room temperature. Cold samples will not flow correctly and can lead to erroneous or invalid results. Several minutes will be required to bring a cold sample to room temperature.
PERFORMANCE CHARACTERISTICS
[Clinical evaluation]
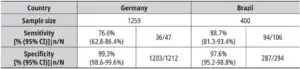
The prospective diagnostic evaluation of STANDARD Q COVID-19 Ag Test was conducted by FIND with collaborators in Germany and Brazil with a total number of enrolled individuals of 1659.The sensitivity and specificity of the STANDARD Q COVID-19 Ag Test was compared to the site-specific RT-PCR method.The pooled sensitivity in Germany was 76.6% (62.8-86.4%) and the pooled specificitywas 99.3% (98.6-99.6%). The sensitivity observed in the testing clinic in Brazil was at 88.7% (81.3 93.4%)and the pooled specificitywas97.6% (95.2-98.8%).
ANALYTICAL PERFORMANCE
- Limit of Detection (LoD)The SARS-CoV-2 positive specimen was prepared by spiking Inactivated SARS-CoV-2 (2019-nCOV) NCCP 43326/2020/Korea strain to SARS-CoV-2 negative nasopharyngeal swab confirmed with PCR. LoD is determined as 3.06 x 102.2 TCID50/ml by testing serially diluted the mock positive specimen.
- Cross-Reactivity& Microbial interferenceThere was no cross-reaction and interference with the potential cross-reacting microorganisms listed below except SARS-COV.


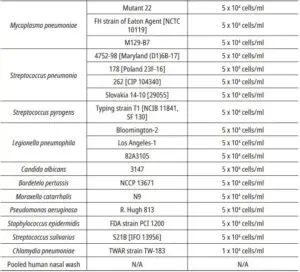
* Human coronavirus HKU1 and Pneumocystis jirovecii (PJP) have not been tested. There can be crossreaction with Human coronavirus HKU1 and Pneumocystis jirovecii (PJP), even though the % identity of the nucleocapsid protein sequence of HKU1 and PJP with the nucleocapsid protein sequence of SARS-CoV-2 was 35.22% and 16.2% which is considered as low homology
3. Exogenous/Endogenous Interference Substances studies: There was no interference for potential interfering substances listed below.
- Exogenous factor


2.Exogenous factor

LIMITATION OF TEST
- The test procedure, precautions and interpretation of results for this test must be followed strictly when testing.
- The test should be used for the detection of SARS-CoV-2 antigen in human nasopharyngeal swab specimens.
- Neither the quantitative value nor the rate of SARS-CoV-2 antigen concentration can be determined by this qualitative test.
- Failure to follow the test procedure and interpretation of test results may adversely affect test performance and/or produce invalid results.
- A negative test result may occur if the level of extracted antigen in a specimen is below the sensitivity of the test or if a poor quality specimen is obtained.
- For more accuracy of immune status, additional follow-up testing using other laboratory methods is recommended.
- The test result must always be evaluated with other data available to the physician.
- A negative result may occur if the concentration of antigen or antibody in a specimen is below the detection limit of the test or if the specimen was collected or transported improperly, therefore a negative test result does not eliminate the possibility of SARS-CoV-2 infection, and should be confirmed by viral culture or an molecular assay or ELISA.
- Positive test results do not rule out co-infections with other pathogens.
- Negative test results are not intended to rule in other coronavirus infection except the SARS-CoV.
- Children tend to shed virus for longer periods of time than adults, which may result in differences in sensitivitybetween adults and children.
- When using VTM, sensitivity can be reduced due to dilution.
- Only Copan UTM, BD UTM and STANDARD™ Transport Medium have been validated with the assay.
BIBLIOGRAPHY
- Clinical management of severe acute respiratory infection when novel coronavirus(nCoV) infection is suspected. Interim guidance. WHO.2020
- Diagnostic detection of Wuhan coronavirus 2019 by real-time RT-PCR.2020
- Diagnosis and treatment of pneumonia caused by new coronavirus (trial version 4) National Health Commission. 2020
Standard Q COVID-19 Ag Test Instructions – Standard Q COVID-19 Ag Test Instructions –
[xyz-ips snippet=”download-snippet”]

