User’s ManualBlood Pressure MonitorModel BP2, BP2A
1. The Basics
This manual contains the instructions necessary to operate the product safely and in accordance with its function and intended use. Observance of this manual is a prerequisite for proper product performance and correct operation and ensures patient and operator safety.
1.1 SafetyWarnings and Cautionary Advices
- Before using the product, please ensure that you have read this manual thoroughly and fully understand corresponding precautions and risks.
- This product has been designed for practical use, but is not a substitute for a visit to the doctor.
- This product is not designed or intended for complete diagnosis of cardiac conditions. This product should never be used as a basis for starting or modifying treatment without independent confirmation by medical examination.
- The data and results displayed on the product are for reference only and cannot be directly used for diagnostic interpretation or treatment.
- Do not attempt self-diagnosis or self-treatment based on the recording results and analysis. Self-diagnosis or self-treatment may lead to deterioration of your health.
- Users should always consult their physician if they notice changes in their health.
- We recommend not to use this product if you have a pacemaker or other implanted products. Follow the advice given by your doctor, if applicable.
- Do not use this product with a defibrillator.
- Never submerge the product in water or other liquids. Do not clean the product with acetone or other volatile solutions.
- Do not drop this product or subject it to strong impact.
- Do not place this product in pressure vessels or gas sterilization product.
- Do not disassemble and modify the product, as this could cause damage, malfunction or impede the operation of the product.
- Do not interconnect the product with other product not described in the Instruction for Use, as this could cause damage or malfunction.
- This product is not intended for use by people (including children) with restricted physical, sensory or mental skills or a lack of experience and/or a lack of knowledge, unless they are supervised by a person who has responsibility for their safety or they receive instructions from this person on how to use the product. Children should be supervised around the product to ensure they do not play with it.
- Do not allow the electrodes of the product to come into contact with other conductive parts (including earth).
- Do not use the product with persons with sensitive skin or allergies.
- DO NOT use this product on infants, toddlers, children or persons who cannot express themselves.
- Do not store the product in the following locations: locations in which the product is exposed to direct sunlight, high temperatures or levels of moisture, or heavy contamination; locations near to sources of water or fire; or locations that are subject to strong electromagnetic influences.
- This product displays changes in the heart rhythm and blood pressure etc. which may have various different causes. These may be harmless, but may also be triggered by illnesses or diseases of differing degree of severity. Please consult a medical specialist if you believe you may have an illness or disease.
- Vital signs measurements, such as those taken with this product, cannot identify all diseases. Regardless of the measurement taken using this product, you should consult your doctor immediately if you experience symptoms that could indicate acute disease.
- Do not self-diagnose or self-medicate on the basis of this product without consulting your doctor. In particular, do not start taking any new medication or change the type and/or dosage of any existing medication without prior approval.
- This product is not a substitute for a medical examination or your heart or other organ function, or for medical electrocardiogram recordings, which require more complex measurements.
- We recommend that you record the ECG curves and other measurements and provide them to your doctor if required.
- Clean the product and cuff with a dry, soft cloth or a cloth dampened with water and a neutral detergent. Never use alcohol, benzene, thinner or other harsh chemicals to clean the product or cuff.
- Avoid tightly folding the cuff or storing the hose tightly twisted for long periods, as such treatment may shorten the life of the components.
- The product and cuff are not water-resistant. Prevent rain, sweat, and water from soiling the product and cuff.
- To measure blood pressure, the arm must be squeezed by the cuff hard enough to temporarily stop blood flow through the artery. This may cause pain, numbness or a temporary red mark to the arm. This condition will appear especially when measurement is repeated successively. Any pain, numbness, or red marks will disappear with time.
- Too frequent measurements can cause injury to the patient due to blood flow interference.
- Consult your physician before using this product on an arm with an arterio-venous (A-V) shunt.
- Consult your physician before using this monitor if you have had a mastectomy or lymph node clearance.
- The pressurization of the CUFF can temporarily cause loss of function of simultaneously used monitoring product on the same limb.
- Consult your physician before using the product if you have severe blood flow problems or blood disorders as cuff inflation can cause bruising.
- Please prevent that operation of the product results in prolonged impairment of the circulation of the blood of the patient.
- Do not apply the cuff on an arm with another medical electrical equipment attached. The equipment may not function properly.
- People who have a severe circulatory deficit in the arm must consult a doctor before using the product, to avoid medical problems.
- Do not self-diagnose the measurement results and start treatment by yourself. Always consult your doctor for evaluation of the results and treatment.
- Do not apply the cuff on an arm with an unhealed wound, as this can cause further injury.
- Do not apply the cuff on an arm receiving an intravenous drip or blood transfusion. It may cause injury or accidents.
- Remove tight-fitting or thick clothing from your arm while taking a measurement.
- If the patients’ arm is outside the specified circumference range that may result in incorrect measurement results.
- The product is not intended for use with neonatal, pregnant, including pre-eclamptic, patients.
- Do not use the product where flammable gases such as anesthetic gases are present. It may cause an explosion.
- Do not use the product in the area of HF surgical equipment, MRI, or CT scanner, or in an oxygen-rich environment.
- The battery intended to be changed only by service personnel with the use of a tool, and replacement by inadequately trained personnel may result in damage or burn.
- The patient is an intended operator.
- Do not carry out the servicing and maintenance while the product is in use.
- The patient can safely use all the functions of the product, and the patient can maintain the product by carefully reading Chapter 7.
- This product emits radio frequencies (RF) in the 2.4 GHz band. DO NOT use this product in locations where RF is restricted, such as on an aircraft. Turn off the Bluetooth feature in this product and remove batteries when in RF restricted areas. For further information on potential restrictions refer to documentation on the Bluetooth usage by the FCC.
- DO NOT use this product with other medical electrical (ME) equipment simultaneously. This may result in incorrect operation of the product and/ or cause an inaccurate blood pressure readings and/ or EKG recordings.
- Sources of electromagnetic disturbance may affect this product (e.g. mobile telephones, microwave cookers, diathermy, lithotripsy, electrocautery, RFID, electromagnetic anti-theft systems, and metal detectors), please try to stay away from them when making measurements.
- The use of accessories and cables other than those specified or provided by manufacture could result in increased electromagnetic emission or decreased electromagnetic immunity of the product and result in improper operation.
- Interpretations made by this product are potential findings, not a complete diagnosis of cardiac conditions. All interpretations should be reviewed by a medical professional for clinical decision-making.
- DO NOT use this product in the presence of flammable anesthetics or drugs.
- DO NOT use this product while charging.
- Remain still while recording an ECG.
- The detectors of ECG have been developed and tested on Lead I and II recordings only.
2. Introduction
2.1 Intended UseThe device is indented to measure blood pressure or electrocardiogram (ECG) in home or healthcare facilities environment.The device is a blood pressure monitor intended for use in measuring blood pressure and pulse rate in adult population.The product is intended to measure, display, store and review adults’ single-channel ECG rhythms and gives some suggested symptoms such as regular beat, irregular beat, low HR and high HR.2.2 ContraindicationsThis product is contraindicated for use in ambulatory environments.This product is contraindicated for use on aircraft.2.3 About the productproduct name: Blood Pressure MonitorProduct model: BP2 (include NIBP+ECG), BP2A (only NIBP)
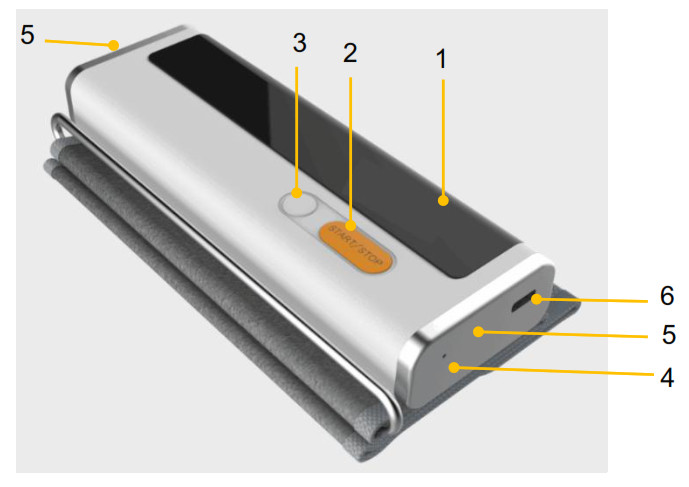
1. LED screen
- Display date, time and power status, etc.
- Display ECG and blood pressure measurement process and results.
2. Start/ Stop button
- Power On/ Off
- Power On:Press the button to power on.
- Power off:Press and hold the button to power off.
- Press to power on the product and press again to start measuring blood pressure.
- Press to power on the product and touch the electrodes to start measuring ECG.
3. Memory button
- Press to review historical data.
4. LED indicator
- Blue light is on: the battery is being charged.
- Blue light is off: the battery is full charged not charging
5. ECG electrode
- Touch them to start measuring ECG with different methods.
6. USB connector
- It connects with charging cable.
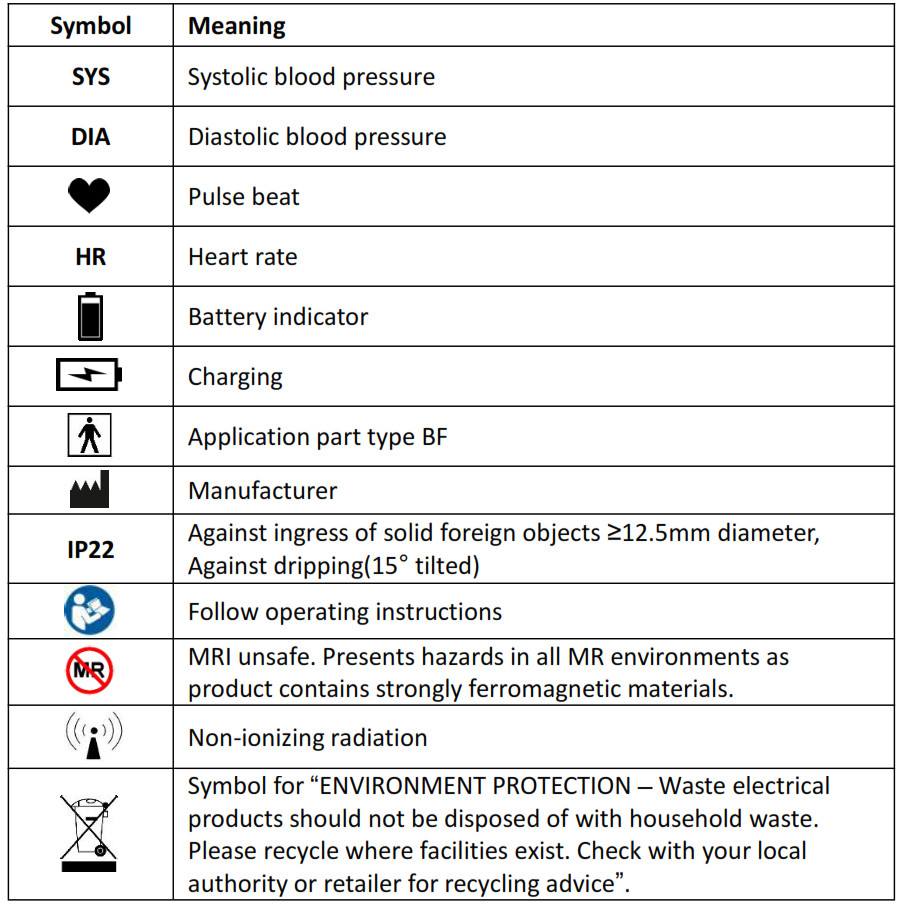
2.4 Symbols
3. Using the Product
3.1 Charge the BatteryUse the USB cable to charge the product. Connect the USB cable to a USB charger or to the PC. A fully charge will need 2 hours. When the battery charged fully the indicator will be blue.The product works in a very low power consumption and one charge usually works for months.On-screen battery symbols which indicate the battery status can be seen on the screen.Note: The product cannot be used during charging, and if choosing a third party charging adaptor, select one that complies with IEC60950 or IEC60601-1.
3.2 Measure Blood Pressure3.2.1 Applying the arm cuff
- Wrap the cuff around the upper arm, about 1 to 2 cm above the inside of the elbow, as shown.
- Place the cuff directly against the skin, as clothing may cause a faint pulse and result in a measurement error.
- Constriction of the upper arm, caused by rolling up a shirtsleeve, may prevent accurate readings.
- Confirm that the artery position mark is line up with the artery.
3.2.2 How to sit correctlyTo take a measurement, you need to be relaxed and comfortably seated. Sit in a chair with your legs uncrossed and your feet flat on the floor. Place your left arm on a table so the cuff is level with your heart.
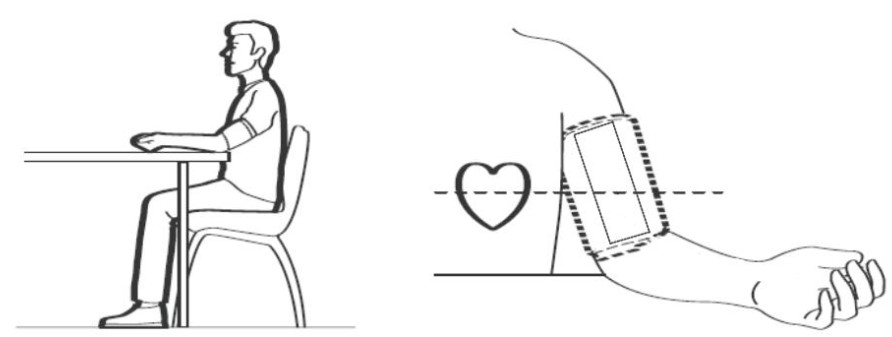
Note:
- The blood pressure can differ between the right arm and the left arm, and the measured blood pressure readings can be different. The Viatom recommends to always use the same arm for measurement. If the blood pressure readings between both arms differ substantially, check with your physician to determine as to which arm to use for your measurements.
- The time is about 5s required for the product to warm from the minimum storage temperature between uses until the product is ready for its intended use when the ambient temperature is 20°C, and the time is about 5s required for the product to cool from the maximum storage temperature between uses until the product is ready for its intended use when the ambient temperature is 20°C.
3.2.3 Measurement process
- Press to power on the product and press again to start measuring blood pressure.
- The product will automatically deflate the cuff slowly during the measurement, a typical measurement takes about 30s.

- The blood pressure readings will scrolling appear in the product when the measurement finished.

- The product will automatically release the cuff gas after the measurement is over.
- Press the button to turn off the power after the measurement, then remove the cuff.
- Press the memory button to review historical data. The blood pressure readings will appear in the product
Note:
- The product has an automatic power shut-off function, which turns off the power automatically in one minute after measurement.
- During the measurement, you should keep still and don’t squeeze the cuff. Stop measuring when the pressure result appear in the product. Otherwise the measurement may be effected and the blood pressure readings may be inaccurate.
- The device can store maximum 100 readings for Blood Pressure data. The oldest record will be overwritten when the 101th readings is coming in. Please uploading data in time.
NIBP Measurement PrincipleThe NIBP measurement way is oscillation method. Oscillation measurement is using automatic inflator pump. When the pressure is high enough to block arterial blood flow, then it would deflate slowly, and record all the change of cuff pressure in the deflation process to calculate blood pressure based on certain algorithm. The computer will judge whether the quality of signal is accurate enough. If the signal is not accurate enough (Such as sudden movement or touch of cuff while measurement), the machine will stop deflating or re-inflate, or abandon this measurement and calculation.The operating steps needed to obtain accurate routine resting blood pressure measurements for the condition hypertension including:— Patient position in normal use, including comfortably seated, legs uncrossed, feet flat on the floor, back and arm supported, middle of the cuff at the level of the right atrium of the heart.— The patient should be relaxed much as possible and should not talk during the measurement procedure.— 5 minutes should elapse before the first reading is taken.— Operator position in normal use.
3.3 Measure ECG3.3.1 Before using ECG
- Before using ECG function, pay attention to the following points in order to obtain precise measurements.
- The ECG electrode must be positioned directly against the skin.
- If your skin or hands are dry, moisten them using a damp cloth before taking the measurement.
- If the ECG electrodes are dirty, remove the dirt using a soft cloth or cotton bud dampened with disinfectant alcohol.
- During the measurement, do not touch your body with the hand with which you are taking the measurement.
- Please note that there must be no skin contact between your right and left hand. Otherwise, the measurement cannot be taken correctly.
- Stay still during the measurement, do not speak, and hold the product still. Movements of any kind will falsify the measurements.
- If possible, take the measurement when sitting and not when standing.
3.3.2 Measurement process
1.Press to power on the product and touch the electrodes to start measuring ECG.→ Method A: Lead I, right hand to left hand → Method B: Lead II, right hand to left abdomen
→ Method B: Lead II, right hand to left abdomen
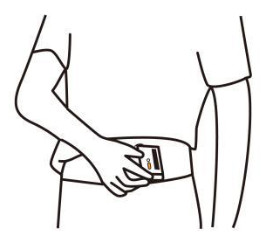
2.Keep touching electrodes gently for 30 seconds.
3.When the bar if fully filled, the product will show the measurement result.

4.Press the memory button to review historical data.
Note:
- Do not press the product too firmly against your skin, which may result in EMG (electromyography) interference.
- The device can store maximum 10 records for ECG data. The oldest record will be overwritten when the 11th record is coming in. Please uploading data in time.
ECG Measurement PrincipleThe product collects the ECG data through the potential difference of the body surface through the ECG electrode, and obtains accurate ECG data after being amplified and filtered, then displays through the screen.Irregular beat: If the change speed of heart rate exceeds a certain threshold during measurement, is judged as irregular heartbeat.High HR: The heart rate > 120 /minLow HR: The heart rate < 50 /minIf the measurement results do not meet the “Irregular beat”, “High HR” and “Low HR”, then judge the “Regular beat”.
3.4 BluetoothThe product Bluetooth will be enabled automatically only when the screen lights up.1) Ensure the product screen is on to keep the product Bluetooth enabled.2) Make sure the phone Bluetooth is enabled.3) Select the product ID from the phone, then the product will be paired successfully with your phone.4) You can export the measured data including SYS, DIS, ECG data to your phone.
Note:
- The Bluetooth technology is based on a radio link that offers fast and reliable data transmissions.The Bluetooth uses a license-free, globally available frequency range in the ISM band-intended to ensure communication compatibility worldwide.
- The pairing and transmitting distance of wireless function is 1.5 meters in the normal. If the wireless communication is delay or failure between the phone and the product, you will try to narrow the distance between the phone and the product.
- The product can pair and transmit with the phone under the wireless coexistence environment (e.g. microwaves, cell phones, routers, radios, electromagnetic anti-theft systems, and metal detectors), but other wireless product may still interface with pairing and transmission between the phone and the product under uncertain environment. If the phone and the product display inconsistent, you may need to change the environment.
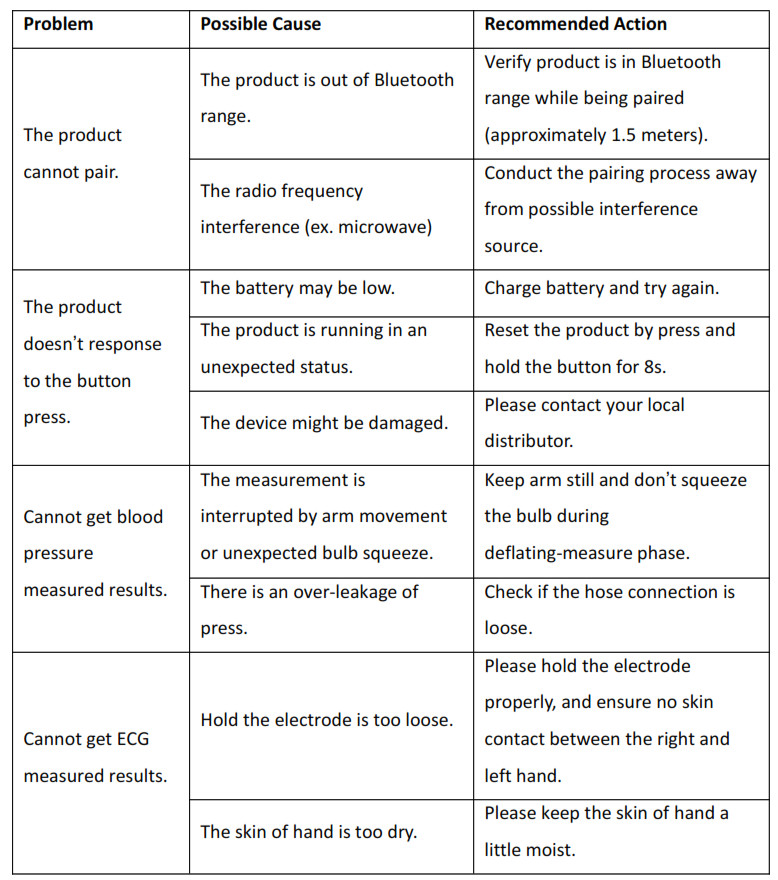
4. Trouble Shooting
5. Accessories

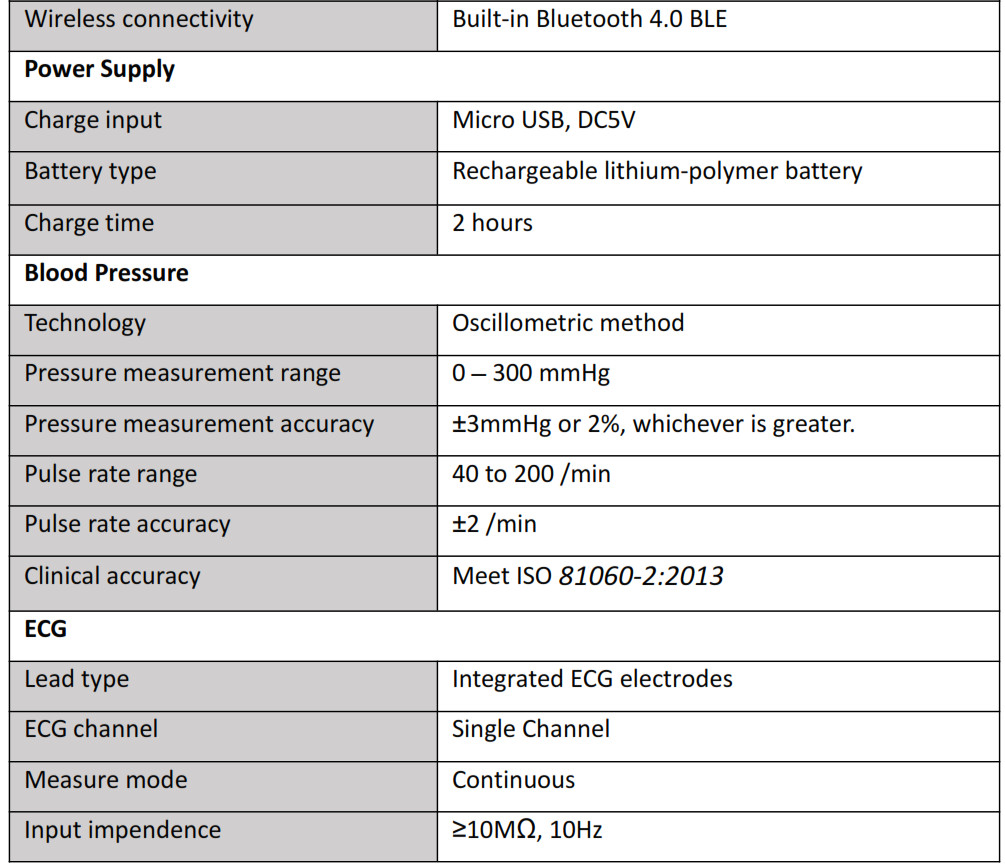
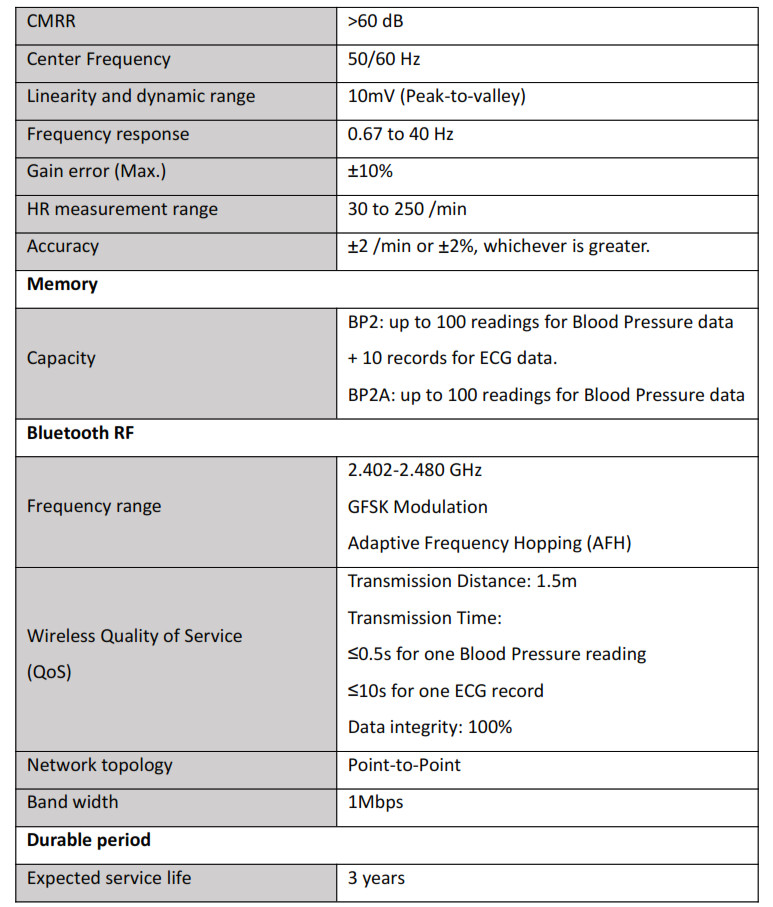
6. Specifications

7. Maintenance and Cleaning
7.1 MaintenanceTo protect your product from damage, please observe the following:
- Store the product and the components in a clean, safe location.
- Do not wash the product and any components or immerse them in water.
- Do not disassemble or attempt to repair the product or components.
- Do not expose the product to extreme temperatures, humidity, dust, or direct sunlight.
- The cuff contains a sensitive air-tight bubble. Handle this carefully and avoid all types of straining through twisting or buckling.
- Clean the product with a soft, dry cloth. Do not use petrol, thinners, or similar solvent. Spots on the cuff can be removed carefully with a damp cloth and soapsuds. The cuff must not be washed!
- Do not drop the instrument or treat it roughly in any way. Avoid strong vibrations.
- Never open the product! Otherwise, the manufacturer calibration becomes invalid!
7.2 CleaningThe product can be repeatedly used. Please clean before reuse as follow:
- Clean the product with a soft, dry cloth with 70% alcohol.
- Do not use petrol, thinners or similar solvent.
- Clean the cuff carefully with cloth soaked 70% alcohol.
- The cuff must not be washed.
- Clean on the product and the arm cuff, and then let it air dry.
7.3 Disposal
![]() Batteries and electronic instruments must be disposed of in accordance with the locally applicable regulations, not with domestics waste.
Batteries and electronic instruments must be disposed of in accordance with the locally applicable regulations, not with domestics waste.
8. FCC Statement
FCC ID: 2ADXK-8621Any Changes or modifications not expressly approved by the party responsible for compliance could void the user’s authority to operate the equipment.This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:(1) This device may not cause harmful interference, and(2) this device must accept any interference received, including interference that may cause undesired operation.
Note: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures:-Reorient or relocate the receiving antenna.-Increase the separation between the equipment and receiver.-Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.-Consult the dealer or an experienced radio/TV technician for help.
The device has been evaluated to meet general RF exposure requirement. The device can be used in portable exposure condition without restriction.
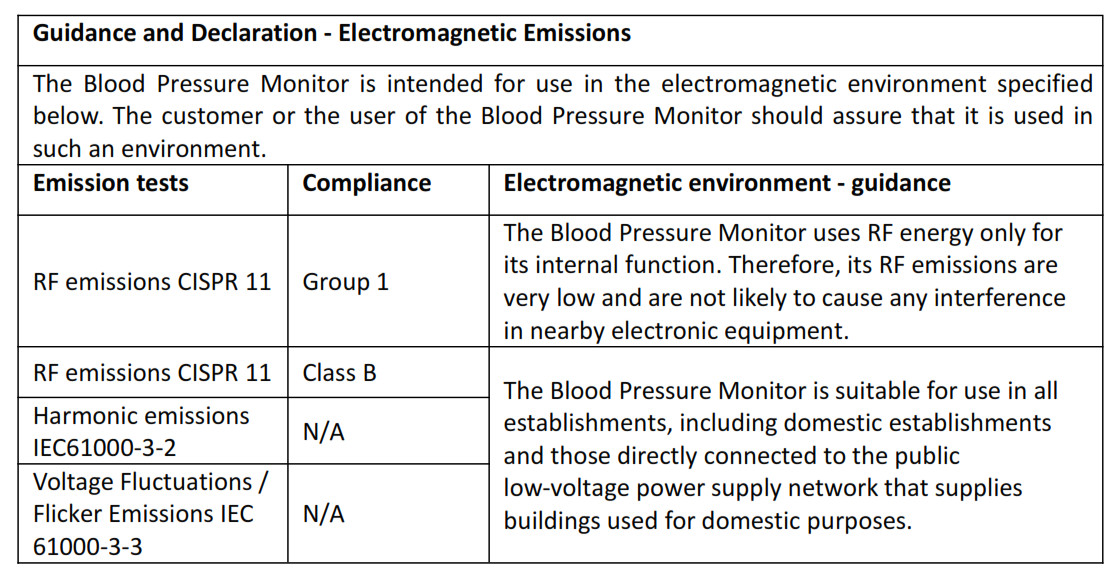
9. Electromagnetic Compatibility
The product meets the requirements of EN 60601-1-2.![]() Warnings and Cautionary Advices
Warnings and Cautionary Advices
- Using accessories other than those specified in this manual may result in increased electromagnetic emission or decreased electromagnetic immunity of the equipment.
- The product or its components should not be used adjacent to or stacked with other equipment.
- The product needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided below.
- Other products may interfere with this product even though they meet the requirements of CISPR.
- When the inputted signal is below the minimum amplitude provided in technical specifications, erroneous measurements could result.
- Portable and mobile communication equipment may affect the performance of this product.
- Other products that have RF transmitter or source may affect this product (e.g. cell phones, PDAs, and PCs with wireless function).
Guidance and Declaration – Electromagnetic Immunity Guidance and Declaration – Electromagnetic Immunity
Guidance and Declaration – Electromagnetic Immunity

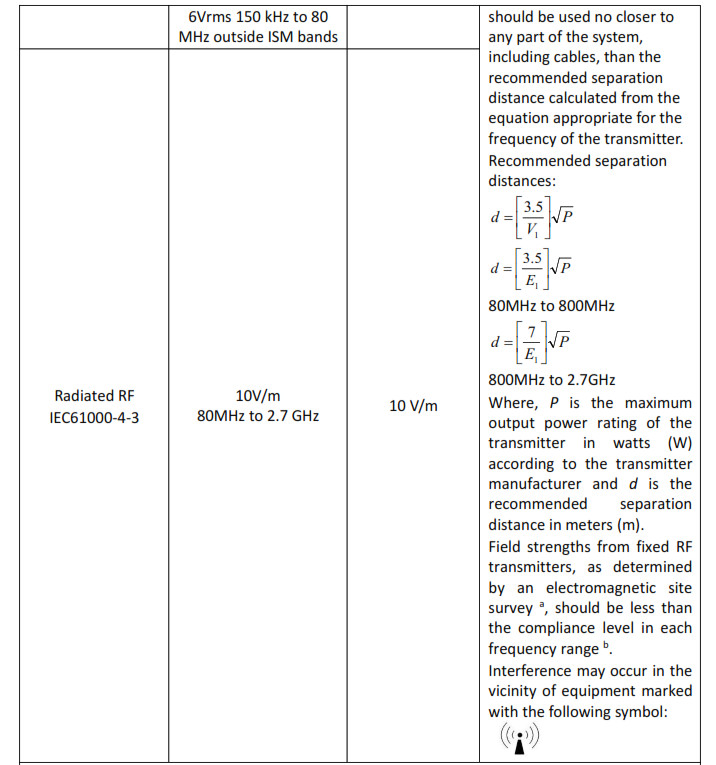
Note 1: At 80 MHz to 800 MHz, the separation distance for the higher frequency range applies.Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
a The ISM (industrial, scientific and medical) bands between 0,15 MHz and 80 MHz are 6,765 MHz to 6,795 MHz; 13,553 MHz to 13,567 MHz; 26,957 MHz to 27,283 MHz; and 40,66 MHz to 40,70 MHz. The amateur radio bands between 0,15 MHz and 80 MHz are 1,8 MHz to 2,0 MHz, 3,5 MHz to 4,0 MHz, 5,3 MHz to 5,4 MHz, 7 MHz to 7,3 MHz, 10,1 MHz to 10,15 MHz, 14 MHz to 14,2 MHz, 18,07 MHz to 18,17 MHz, 21,0 MHz to 21,4 MHz, 24,89 MHz to 24,99 MHz, 28,0 MHz to 29,7 MHz and 50,0 MHz to 54,0 MHz.
b The compliance levels in the ISM frequency bands between 150 kHz and 80 MHz and in the frequency range 80 MHz to 2,7 GHz are intended to decrease the likelihood that mobile/portable communications equipment could cause interference if it is inadvertently brought into patient areas. For this reason, an additional factor of 10/3 has been incorporated into the formulae used in calculating the recommended separation distance for transmitters in these frequency ranges.
c Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM, and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Blood Pressure Monitor is used exceeds the applicable RF compliance level above, the Blood Pressure Monitor should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the Blood Pressure Monitor.
d Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
 Shenzhen Viatom Technology Co., Ltd.4E,Building 3, Tingwei Industrial Park, No.6Liufang Road, Block 67, Xin’an Street,Baoan District, Shenzhen 518101 GuangdongChinawww.viatomtech.com
Shenzhen Viatom Technology Co., Ltd.4E,Building 3, Tingwei Industrial Park, No.6Liufang Road, Block 67, Xin’an Street,Baoan District, Shenzhen 518101 GuangdongChinawww.viatomtech.com
PN:255-01761-00 Version: A Oct, 2019
Viatom Blood Pressure Monitor BP2 & BP2A User Manual – Viatom Blood Pressure Monitor BP2 & BP2A User Manual –
[xyz-ips snippet=”download-snippet”]



