SARS-CoV-2 Rapid Antigen Test
INTENDED USE
Wollerau Pharma SARS-CoV-2 Rapid Antigen Test Rapid CAT is intended for the qualitative detection of antigens from severe acute respiratory syndrome as-sociated coronavirus 2 (SARS-CoV-2) in a clinical specimen. The product is intended for professional use in laboratory and Point of Care environments.
SUMMARY
The novel coronaviruses belong to the~ genus. COVID-19 is an acute respiratory infectious disease. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, most commonly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases. Standard recommendations to prevent infection spread include regular hand washing, covering mouth and nose when coughing and sneezing. Avoid close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing.
TEST PRINCIPLE
Wollerau Pharma SARS-CoV-2 Rapid Antigen Test Rapid CAT is a double antibody-sandwich, qualitative membrane-based immunoassay In-vitro diagnostic medical device. The kit is designed to detect nucleocapsid antigen from the SARS-CoV-2 in nasopharyngeal swab or oropharyngeal swab from patients who are suspected of being COVID-19 positive. The SARS-CoV-2 antigens present in the specimen react with anti- SARS-CoV-2 antibody-coated particles in the test cassette. The mixture then migrates upward on the membrane by capillary action and reacts with the precoated antibody in the test line region. If the specimen contains SARSCoV-2 antigens, a coloured line will appear in the test line region. If the specimen does not contain SARS-CoV-2 antigens, no coloured line will appear in the test line region, indicating a negative result. To serve as a procedural control, a coloured line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
STORAGE INSTRUCTIONS
- Store the kit at room temperature or refrigerated {2-30°C).
- Do not freeze.
- The kit has a shelf-life of 18 months.
REAGENTS
PRECAUTIONS AND WARNINGS
- Do not re-use the test kit.
- Do not use the test kit if the pouch is damaged or the seal is broken.
- Do not use the extraction buffer tube of a different lot.
- Do not smoke, drink or eat while handling sample.
- Wear personal protective equipment, such as gloves and lab coats when handling kit reagents. Wash hands thoroughly after the tests are done.
- Clean up spills thoroughly using an appropriate disinfectant.
- Handle all samples as if they contain infectious agents.
- Observe established precautions against microbiological hazards throughout testing procedures.
- Dispose all samples and materials used to perform the test as biohazardous waste. Laboratory chemical and biohazardous wastes must be handled and discarded in accordance with all local, state and national regulations.
CONTENTS OF THE KIT
20 x Test Cassette, 20 x Sterilised nasopharyngeal swab, 20 x reagent in tube with dropper, Instructions for use.One test cassette contains: a membrane strip coated with anti-SARS- CoV2monoclonal antibody on the test line and a dye pad which contains colloidal gold coupled with SARS-CoV-2 monoclonal antibody.
Materials not included but required: Gloves, Timer
SAMPLE REQUIREMENTS
Specimens obtained early during symptom onset will contain the highest viral titers. Specimens obtained after 5 days of symptoms are more likely to produce negative results when compared to an RT-PCR assay. Inadequate specimen, improper specimen handling and/or transport may yield a false negative result; therefore, training in specimen collection is highly recommended due to the importance of specimen quality for generating accurate test results.
PREPARING FOR A TEST
- Check the expiry date on pouch. Do not use the test, if the expiry date has passed.
- Open the foil pouch and remove the test device. Use the test immediately after opening the pouch.
SAMPLE COLLECTION
Insert mini-tip swab with a flexible shaft (wire or plastic) through the nostril parallel to the palate (not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx. Swab should reach depth equal to distance from nostrils to outer opening of the ear. Gently rub and roll the swab. Leave swab in place for several seconds to absorb secretions. Slowly remove swab while rotating it. Specimens can be collected from both sides using the same swab, but it is not necessary to collect specimens from both sides if the mini-tip is saturated with fluid from the first collection. If a deviated sep-tum or blockage creates difficulty in obtaining the specimen from one nostril, use the same swab to obtain the specimen from the other nostril.
SPECIMEN PREPARATION
- Open the lid of the tube containing the buffer solution.
- Insert the swab into the tube.
- Rotate the swab inside the tube for one minute.
- Close the extraction tube with the dropper back on tightly.
- Use dropper to transfer 3 drops of the specimen with reagent to the specimen well of the test cassette.
- Start the timer and read the results after 15 – 30 minutes.
SAMPLE TRANSPORT AND STORAGE
Freshly collected specimens should be prepared as soon as possible and no later than one hour after specimen collection. Specimen already prepared may be stored at 2-8°C for no more than 24 hours. If long-term storage is required, store at -70 °C and avoid repeated freeze-thaw cycles.
RESULTS
A total of three detection lines are possible, with the control (C) line appearing when sample has been flowed through the cassette.
- Negative Result: One coloured line appears in the control line region (C). No line appears in the test region (Tl
- Positive Result: Two coloured lines appear. One coloured line appears in the control line region (C) and another line adjacent appears in the test region (T).
- Invalid Result: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
INTERNAL QUALITY CONTROL PROCEDURE
Each Test Cassette device has a built-in control. A red coloured line in the detection window at the Control line can be considered an internal positive procedural control. The Control line will appear if the test procedure has been correctly performed. If the Control line does not appear, the test is invalid and a new test must be performed. If the problem persists, please contact your local vendor or Wollerau Pharma for technical support.
WARNINGS AND PRECAUTIONS
- For professional In-vitro diagnostic use only.
- Follow-up testing with a molecular diagnostic should be considered.
- Results from antigen testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
- Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status.
- Positive results do not rule out bacterial infection or co-infection with other viruses.
- This test must be administered by a medical professional.
LIMITATIONS
- The test procedure, precautions and interpretation of results for this test must be followed strictly when testing.
- The test should be used for the detection of SARS-CoV-2 antigen in human nasopharyngeal swab samples.
- This is a qualitative test, therefore quantitative values of SARSCoV-2 antigen concentration cannot be determined.
- The immune response cannot be assessed with this test and needs other testing methods. The test result should not be used as a sole basis for treatment or patient management decisions and should be considered in the context of the patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.
- A negative result may occur if the concentration of antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly. Therefore a negative test result does not eliminate the possibility of SARS-CoV-2 infection and should be confirmed by viral culture or a molecular assay or ELISA, if necessary for patient management.
- Positive test results do not rule out co-infections with other pathogens.
- Positive test results do not differentiate between SARS-CoV-2 and SARS-CoV.
- Negative test results are not intended to rule in or rule out other coronavirus infection.
SPECIFIC PERFORMANCE DATA
The sensitivity of the Wollerau Pharma SARS-CoV-2 Rapid Antigen Test Rapid CAT for rapid detection of SARS-CoV-2 antigen was established in prospective, randomized, single blinded studies conducted during the SARS-CoV-2 pandemic in China and Budapest. A total of 80 positive samples were tested using the Wollerau Pharma SARS-CoV-2 Rapid Antigen Test Rapid CAT. These samples consisted of nasopharyngeal swabs from symptomatic and asymptomatic patients. The specificity of the Wollerau Pharma SARS-CoV-2 Rapid Antigen Test Rapid CAT was tested using 148 negative samples. The sensitivity and specificity of the Wollerau Pharma SARS-CoV-2 Rapid Antigen Test Rapid CAT was compared to commercialized molecular assays.
TEST SENSITIVITY AND SPECIFICITY
The clinical trial was performed at the Hubei Provincial Centre for Disease Control and Prevention on 9th October, 2020 with 68 RT-PCR positive samples and 140 RT-PCR negative samples.
ANALYTICAL PERFORMANCE
- Limit of detection (LoD):The study used the “SARS-CoV-2 (2019-nCOV) strain. The titer of cultured virus was confirmed by PCR. The LoD is 35 ng/ml
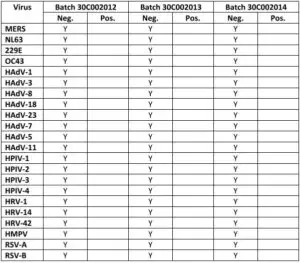
- Cross reactivity:There was no cross-reaction with potential cross-reactive substances except SARS coronavirus. Cross reactivity testing with SARS-CoV-2 negative samples:

- Endogenous/ exogenous interference substances studies:There was no interference on the test result from potentially interfering substances listed below. SARS-CoV-2 positive and negative samples were tested. a) Results from interference testing with SARS-CoV-2 negative samples:

SYMBOLS
Wollerau Pharma SARS-CoV-2 Rapid Antigen Test Instructions for Use – Wollerau Pharma SARS-CoV-2 Rapid Antigen Test Instructions for Use –
[xyz-ips snippet=”download-snippet”]
