WREN COVID-19 Saliva Test Collection Kit DTC Instruction Manual
Additional Information
(fact sheets, additional instructions), can be downloaded at www.wrencovidtesting.com. For any questions, please contact Wren Laboratories at [email protected]
- For use by individuals 18 years and older (self-collected)
- For use by individuals 14 years and older (self-collected under adult supervision)
- For use by individuals 5 years and older (collected with adult assistance) Follow directions for use.Do NOT ingest the stabilizing buffer. Keep out of reach of children. Avoid contact with skin and eyes.If the stabilizing buffer comes in contact with the body, rinse with water. If irritation persists, seek medical advice. It is recommended to use gloves and eye wear when performing the collection procedure.
WARNING
This product has not been FDA cleared or approved, but has been authorized for emergency use by FDA under an EUA;
This product has been authorized only for the home collection and maintenance of saliva specimens as an aid in detection of nucleir acid from SARS-CoV-2, not for any other viruses or pathogens; and, The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of medical devices under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
To view Wren Laboratories complete Terms & Conditions, please visit: www.wrencovidtesting.com/termsandconditions
For more information about the Wren Covid-19 PCR Test visit www.wrencovidtesting.com Wren Laboratories, 688 East Main Street, Branford, CT 06405 Tel: (203) 208-3464 © Wren Laboratories. All rights reserved. For In Vitro Diagnostic Use. For Use Under Emergency Use Authorization Only.
Your test kit includes the following components:
- Shipping box
- Pre-paid/pre-addressed return label
- Fact Sheet for Individuals
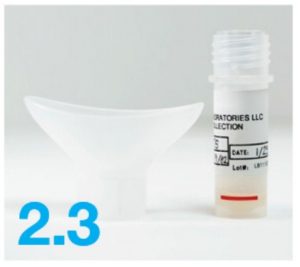
- Saliva collection device
- 2 sealing strips
- A Biohazard Specimen Bag with absorbent material.
Do not discard. Required for return shipping.
Instructions for use
Step 1: Register Your Test Kit.
Use your smartphone camera to scan the QR Code on the enclosed tube. This will take you to a website where you will be prompted to enter your information as directed. (In case you cannot use a smartphone to enter your personal data and complete the online requisition form, please visit: www.wrencovidtesting.com/start).
STOP Do not start the saliva collection process before you have completed the registration of your test kit.
Once registration is complete, open your test kit and place all contents on a clean, dry surface. Now go to Step 2.
Note: An instructional video demonstrating the saliva collection process is available online (www.wrencovidtesting.com)
Do not eat, drink (even water), brush your teeth, or smoke, for 30 minutes prior to collecting your saliva sample. If you have trouble collecting enough saliva gently rub the outside of your cheeks, just behind your back teeth while performing chewing motions with your mouth.
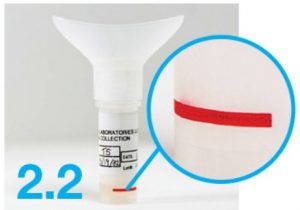
Collect enough saliva to fill at least up to the red line as marked on the tube.
Step 2: Collecting Saliva Sample
Your sample must be returned/shipped on the same day it was collected to ensure timely receipt at the laboratory for processing.
- Attach the funnel to the tube (screw it on gently) until fully attached.

- Collect your saliva sample. Fill the N tube until the saliva (not including bubbles) is just above the red line. / Do not overfill.

- Remove the funnel from the tube and discard the funnel.

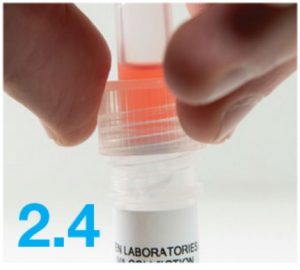
- Firmly screw the cap down onto the tube. This will release the red solution and seal the tube. You will know this has worked because your saliva will change color. Firmly tighten the cap to ensure the cap and tube are completely sealed.WARNING: DO NOT OPEN OR REMOVE CAP ONCE SEALED

- Shake the tube gently for 10 seconds. This will guarantee your sample mixes thoroughly with the stabilizing solution.

- Write your initials, date of birth and today’s date on the collection tube label using either pen or pencil.

- Place the collection tube into the Biohazard Specimen Bag with the absorbent material. Do not remove any absorbent material from the bag. Seal the Biohazard Specimen Bag. Wash and dry your hands thoroughly before moving on to step 3.

Step 3: Ship Sample via FedEx
- Place the Specimen Bag with the collected sample into the box.

- Attach the pre-paid return shipping label to the top of the box. Write your name under the order number.

- Important! Record your FEDEX tracking information in the box below before shipment. Keep this document for your records.

- Seal the Box using the two enclosed strips on each side of the box. Deliver the box to a FedEx drop box or to a FedEx location.

- 3.5 Your results will be emailed to you. If positive, presumptive positive or invalid, you will be contacted by a HCP for a recommended course of care and appropriate follow-up action.
Wren Saliva Test Kit Sleeve Templates
Your kit includes:
- Instructions
- Mouthpiece
- Collection tube
- Cap
- Biohazard bag with absorbent material
- 2 sealing strips
- Fact Sheet for Individuals
- Return shipping box
- Pre-paid/pre-addressed FedEx return label
For IVD use. For use under emergency use authorization only.
WREN Laboratories offers this product with a FDA Emergency Use Authorization (EUA). This means that this product has not been FDA cleared or approved. but has been authorized for emergency use by FDA under an EUA.
This product has been authorized only for the home collection and maintenance of saliva specimens as an aid in detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of medical devices under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act. 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Expiration Date: Store at room temperature.
- Register your kit online

- Easily Collect Your Saliva Sample and shop

- Receive Your Results Fast and Online

[xyz-ips snippet=”download-snippet”]